Modeling the blood-brain barrier using stem cell sources
- PMID: 23305164
- PMCID: PMC3564868
- DOI: 10.1186/2045-8118-10-2
Modeling the blood-brain barrier using stem cell sources
Abstract
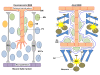
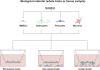
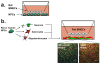
The blood-brain barrier (BBB) is a selective endothelial interface that controls trafficking between the bloodstream and brain interstitial space. During development, the BBB arises as a result of complex multicellular interactions between immature endothelial cells and neural progenitors, neurons, radial glia, and pericytes. As the brain develops, astrocytes and pericytes further contribute to BBB induction and maintenance of the BBB phenotype. Because BBB development, maintenance, and disease states are difficult and time-consuming to study in vivo, researchers often utilize in vitro models for simplified analyses and higher throughput. The in vitro format also provides a platform for screening brain-penetrating therapeutics. However, BBB models derived from adult tissue, especially human sources, have been hampered by limited cell availability and model fidelity. Furthermore, BBB endothelium is very difficult if not impossible to isolate from embryonic animal or human brain, restricting capabilities to model BBB development in vitro. In an effort to address some of these shortcomings, advances in stem cell research have recently been leveraged for improving our understanding of BBB development and function. Stem cells, which are defined by their capacity to expand by self-renewal, can be coaxed to form various somatic cell types and could in principle be very attractive for BBB modeling applications. In this review, we will describe how neural progenitor cells (NPCs), the in vitro precursors to neurons, astrocytes, and oligodendrocytes, can be used to study BBB induction. Next, we will detail how these same NPCs can be differentiated to more mature populations of neurons and astrocytes and profile their use in co-culture modeling of the adult BBB. Finally, we will describe our recent efforts in differentiating human pluripotent stem cells (hPSCs) to endothelial cells with robust BBB characteristics and detail how these cells could ultimately be used to study BBB development and maintenance, to model neurological disease, and to screen neuropharmaceuticals.
Figures


Similar articles
-
A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources.Sci Rep. 2014 Feb 24;4:4160. doi: 10.1038/srep04160. Sci Rep. 2014. PMID: 24561821 Free PMC article.
-
An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells.J Neurochem. 2017 Mar;140(6):874-888. doi: 10.1111/jnc.13923. Epub 2017 Feb 14. J Neurochem. 2017. PMID: 27935037 Free PMC article.
-
In Vitro Modeling of Blood-Brain Barrier with Human iPSC-Derived Endothelial Cells, Pericytes, Neurons, and Astrocytes via Notch Signaling.Stem Cell Reports. 2017 Mar 14;8(3):634-647. doi: 10.1016/j.stemcr.2017.01.023. Epub 2017 Feb 23. Stem Cell Reports. 2017. Retraction in: Stem Cell Reports. 2018 Feb 13;10(2):674. doi: 10.1016/j.stemcr.2018.01.033. PMID: 28238797 Free PMC article. Retracted.
-
Neurological diseases at the blood-brain barrier: Stemming new scientific paradigms using patient-derived induced pluripotent cells.Biochim Biophys Acta Mol Basis Dis. 2020 Apr 1;1866(4):165358. doi: 10.1016/j.bbadis.2018.12.009. Epub 2018 Dec 26. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 30593893 Review.
-
Applications and Considerations for Microfluidic Systems To Model the Blood-Brain Barrier.ACS Appl Bio Mater. 2023 Sep 18;6(9):3617-3632. doi: 10.1021/acsabm.3c00364. Epub 2023 Aug 15. ACS Appl Bio Mater. 2023. PMID: 37582179 Free PMC article. Review.
Cited by
-
Blood-brain barrier dysfunction following traumatic brain injury.Metab Brain Dis. 2015 Oct;30(5):1093-104. doi: 10.1007/s11011-015-9651-7. Epub 2015 Jan 28. Metab Brain Dis. 2015. PMID: 25624154 Review.
-
Generation of Brain Microvascular Endothelial-like Cells from Human iPS Cell-Derived Endothelial Progenitor Cells Using TGF-β Receptor Inhibitor, Laminin 511 Fragment, and Neuronal Cell Culture Supplements.Pharmaceutics. 2022 Dec 2;14(12):2697. doi: 10.3390/pharmaceutics14122697. Pharmaceutics. 2022. PMID: 36559191 Free PMC article.
-
Molecular chaperones in the brain endothelial barrier: neurotoxicity or neuroprotection?FASEB J. 2019 Nov;33(11):11629-11639. doi: 10.1096/fj.201900895R. Epub 2019 Jul 26. FASEB J. 2019. PMID: 31348679 Free PMC article. Review.
-
TGF-β and SHH Regulate Pluripotent Stem Cell Differentiation into Brain Microvascular Endothelial Cells in Generating an In Vitro Blood-Brain Barrier Model.Bioengineering (Basel). 2023 Sep 27;10(10):1132. doi: 10.3390/bioengineering10101132. Bioengineering (Basel). 2023. PMID: 37892862 Free PMC article.
-
Animal models in neuroscience with alternative approaches: Evolutionary, biomedical, and ethical perspectives.Animal Model Exp Med. 2024 Dec;7(6):868-880. doi: 10.1002/ame2.12487. Epub 2024 Oct 7. Animal Model Exp Med. 2024. PMID: 39375824 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

