Reduced indinavir exposure during pregnancy
- PMID: 23305215
- PMCID: PMC3769674
- DOI: 10.1111/bcp.12078
Reduced indinavir exposure during pregnancy
Abstract
Aim: To describe the pharmacokinetics and safety of indinavir boosted with ritonavir (IDV/r) during the second and third trimesters of pregnancy and in the post-partum period.
Methods: IMPAACT P1026s is an on-going, prospective, non-blinded study of antiretroviral pharmacokinetics (PK) in HIV-infected pregnant women with a Thai cohort receiving IDV/r 400/100 mg twice daily during pregnancy through to 6-12 weeks post-partum as part of clinical care. Steady-state PK profiles were performed during the second (optional) and third trimesters and at 6-12 weeks post-partum. PK targets were the estimated 10(th) percentile IDV AUC (12.9 μg ml(-1)h) in non-pregnant historical Thai adults and a trough concentration of 0.1 μg ml(-1), the suggested minimum target.
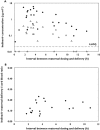
Results: Twenty-six pregnant women were enrolled; thirteen entered during the second trimester. Median (range) age was 29.8 (18.9-40.8) years and weight 60.5 (50.0-85.0) kg at the third trimester PK visit. The 90% confidence limits for the geometric mean ratio of the indinavir AUC(0,12 h) and Cmax during the second trimester and post-partum (ante : post ratios) were 0.58 (0.49, 0.68) and 0.73 (0.59, 0.91), respectively; third trimester/post-partum AUC(0,12 h) and Cmax ratios were 0.60 (0.53, 0.68) and 0.63 (0.55, 0.72), respectively. IDV/r was well tolerated and 21/26 women had a HIV-1 viral load < 40 copies ml(-1) at delivery. All 26 infants were confirmed HIV negative.
Conclusion: Indinavir exposure during the second and third trimesters was significantly reduced compared with post-partum and ∼30% of women failed to achieve a target trough concentration. Increasing the dose of IDV/r during pregnancy to 600/100 mg twice daily may be preferable to ensure adequate drug concentrations.
Keywords: HIV; antiretrovirals; pregnancy; prevention of mother-to-child transmission.
© 2013 The Authors. British Journal of Clinical Pharmacology © 2013 The British Pharmacological Society.
Figures
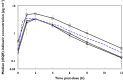
 , second trimester;
, second trimester;  , third trimester;
, third trimester;  , post-partum;
, post-partum;  , non-pregnant
, non-pregnant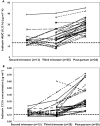
References
-
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43:1071–1087. - PubMed
-
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. September 14, 2011; pp 1–207. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf (last accessed 5 June 2012)
-
- Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, Elgie C, Holland DT, Smith E, Tuomala R, Cotter A, Read JS. Reduced lopinavir exposure during pregnancy. Aids. 2006;20:1931–1939. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ, Jr, Feinberg JE, Balfour HH, Jr, Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- 1 U01 AI068616/AI/NIAID NIH HHS/United States
- 5U01 AI41110/AI/NIAID NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- N01-DK-9-001/HHSN267200800001C/DK/NIDDK NIH HHS/United States
- U01AI068632/AI/NIAID NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

