Impedance-based cell monitoring: barrier properties and beyond
- PMID: 23305242
- PMCID: PMC3560213
- DOI: 10.1186/2045-8118-10-5
Impedance-based cell monitoring: barrier properties and beyond
Abstract
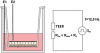
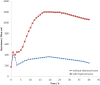
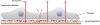
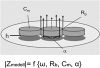
In multicellular organisms epithelial and endothelial cells form selective permeable interfaces between tissue compartments of different chemical compositions. Tight junctions which connect adjacent cells, control the passage of molecules across the barrier and, in addition, facilitate active transport processes. The cellular barriers are not static but can be deliberately modulated by exposure to specific external stimuli. In vitro models representing the essential absorption barriers of the body are nowadays available, thus allowing investigation of the parameters that control permeability as well as transport processes across those barriers. Independent of the origin of the barrier forming cells, techniques are needed to quantify their barrier integrity. One simple assay is to measure the permeability for given hydrophilic substrates possessing different molecular weights like sucrose or dextrans. However, this technique is time-consuming and labor-intensive. Moreover, radioactive or fluorescently-labeled substrates are needed to allow easy analytical detection. Finally, if transport processes are investigated, the standard permeant may interfere with the transport process under investigation or might even alter the barrier integrity by itself. Thus, independent, non-invasive techniques are needed to quantify the barrier integrity continuously during the experiment. Such techniques are available and are mainly based on the measurement of the transendothelial or transepithelial electrical resistance (TEER) of barrier forming cells grown on porous membranes. Simple devices using two sets of electrodes (so-called Voltohmeters) are widely used. In addition, an easy-to-use physical technique called impedance spectroscopy allows the continuous analysis of both the TEER and the electrical capacitance giving additional information about the barrier properties of cells grown on permeable membranes. This technique is useful as a quality control for barrier forming cells. Another impedance-based approach requires cells to be grown directly on solid, micro-structured electrodes. Here, we will discuss the physical background of the different techniques; advantages, disadvantages, and applications will be scrutinized. The aim is to give the reader a comprehensive understanding concerning the range and limits of the application, mainly focusing on endothelial cells.
Figures


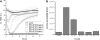




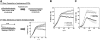

References
-
- Schirmacher A, Winters S, Fischer S, Goeke J, Galla HJ, Kullnick U, Ringelstein EB, Stogbauer F. Electromagnetic fields (1.8 GHz) increase the permeability to sucrose of the blood–brain barrier in vitro. Bioelectromagnetics. 2000;21:338–345. doi: 10.1002/1521-186X(200007)21:5<338::AID-BEM2>3.0.CO;2-Q. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

