Improved manganese-oxidizing activity of DypB, a peroxidase from a lignolytic bacterium
- PMID: 23305326
- PMCID: PMC3631457
- DOI: 10.1021/cb300608x
Improved manganese-oxidizing activity of DypB, a peroxidase from a lignolytic bacterium
Abstract
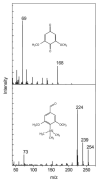
DypB, a dye-decolorizing peroxidase from the lignolytic soil bacterium Rhodococcus jostii RHA1, catalyzes the peroxide-dependent oxidation of divalent manganese (Mn(2+)), albeit less efficiently than fungal manganese peroxidases. Substitution of Asn246, a distal heme residue, with alanine increased the enzyme's apparent k(cat) and k(cat)/K(m) values for Mn(2+) by 80- and 15-fold, respectively. A 2.2 Å resolution X-ray crystal structure of the N246A variant revealed the Mn(2+) to be bound within a pocket of acidic residues at the heme edge, reminiscent of the binding site in fungal manganese peroxidase and very different from that of another bacterial Mn(2+)-oxidizing peroxidase. The first coordination sphere was entirely composed of solvent, consistent with the variant's high K(m) for Mn(2+) (17 ± 2 mM). N246A catalyzed the manganese-dependent transformation of hard wood kraft lignin and its solvent-extracted fractions. Two of the major degradation products were identified as 2,6-dimethoxybenzoquinone and 4-hydroxy-3,5-dimethoxybenzaldehyde, respectively. These results highlight the potential of bacterial enzymes as biocatalysts to transform lignin.
Figures

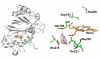


References
-
- Stocker M. Biofuels and biomass-to-liquid fuels in the biorefinery: catalytic conversion of lignocellulosic biomass using porous materials. Angew. Chem. Int. Ed. Engl. 2008;47:9200–9211. - PubMed
-
- Zakzeski J, Bruijnincx PC, Jongerius AL, Weckhuysen BM. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010;110:3552–3599. - PubMed
-
- Tien M, Kirk TK. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium burds. Science. 1983;221:661–663. - PubMed
-
- Glenn JK, Gold MH. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch. Biochem. Biophys. 1985;242:329–341. - PubMed
-
- Leonowicz A, Matuszewska A, Luterek J, Ziegenhagen D, Wojtas-Wasilewska M, Cho NS, Hofrichter M, Rogalski J. Biodegradation of lignin by white rot fungi. Fungal. Genet. Biol. 1999;27:175–185. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

