Postnatally-transmitted HIV-1 Envelope variants have similar neutralization-sensitivity and function to that of nontransmitted breast milk variants
- PMID: 23305422
- PMCID: PMC3564832
- DOI: 10.1186/1742-4690-10-3
Postnatally-transmitted HIV-1 Envelope variants have similar neutralization-sensitivity and function to that of nontransmitted breast milk variants
Abstract
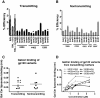
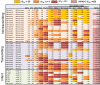
Background: Breastfeeding is a leading cause of infant HIV-1 infection in the developing world, yet only a minority of infants exposed to HIV-1 via breastfeeding become infected. As a genetic bottleneck severely restricts the number of postnatally-transmitted variants, genetic or phenotypic properties of the virus Envelope (Env) could be important for the establishment of infant infection. We examined the efficiency of virologic functions required for initiation of infection in the gastrointestinal tract and the neutralization sensitivity of HIV-1 Env variants isolated from milk of three postnatally-transmitting mothers (n = 13 viruses), five clinically-matched nontransmitting mothers (n = 16 viruses), and seven postnatally-infected infants (n = 7 postnatally-transmitted/founder (T/F) viruses).
Results: There was no difference in the efficiency of epithelial cell interactions between Env virus variants from the breast milk of transmitting and nontransmitting mothers. Moreover, there was similar efficiency of DC-mediated trans-infection, CCR5-usage, target cell fusion, and infectivity between HIV-1 Env-pseudoviruses from nontransmitting mothers and postnatal T/F viruses. Milk Env-pseudoviruses were generally sensitive to neutralization by autologous maternal plasma and resistant to breast milk neutralization. Infant T/F Env-pseudoviruses were equally sensitive to neutralization by broadly-neutralizing monoclonal and polyclonal antibodies as compared to nontransmitted breast milk Env variants.
Conclusion: Postnatally-T/F Env variants do not appear to possess a superior ability to interact with and cross a mucosal barrier or an exceptional resistance to neutralization that define their capability to initiate infection across the infant gastrointestinal tract in the setting of preexisting maternal antibodies.
Figures

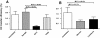
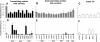

Similar articles
-
Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1.J Virol. 2015 Oct;89(19):9952-61. doi: 10.1128/JVI.01560-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202232 Free PMC article. Clinical Trial.
-
Infant transmitted/founder HIV-1 viruses from peripartum transmission are neutralization resistant to paired maternal plasma.PLoS Pathog. 2018 Apr 19;14(4):e1006944. doi: 10.1371/journal.ppat.1006944. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29672607 Free PMC article.
-
Mutations that confer resistance to broadly-neutralizing antibodies define HIV-1 variants of transmitting mothers from that of non-transmitting mothers.PLoS Pathog. 2021 Apr 2;17(4):e1009478. doi: 10.1371/journal.ppat.1009478. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33798244 Free PMC article.
-
HIV transmission.Cold Spring Harb Perspect Med. 2012 Nov 1;2(11):a006965. doi: 10.1101/cshperspect.a006965. Cold Spring Harb Perspect Med. 2012. PMID: 23043157 Free PMC article. Review.
-
HIV-1 reservoirs in breast milk and challenges to elimination of breast-feeding transmission of HIV-1.Sci Transl Med. 2012 Jul 18;4(143):143sr3. doi: 10.1126/scitranslmed.3003327. Sci Transl Med. 2012. PMID: 22814853 Review.
Cited by
-
The Role of Maternal HIV Envelope-Specific Antibodies and Mother-to-Child Transmission Risk.Front Immunol. 2017 Sep 4;8:1091. doi: 10.3389/fimmu.2017.01091. eCollection 2017. Front Immunol. 2017. PMID: 28928750 Free PMC article. Review.
-
Disentangling the impact of within-host evolution and transmission dynamics on the tempo of HIV-1 evolution.AIDS. 2015 Jul 31;29(12):1549-56. doi: 10.1097/QAD.0000000000000731. AIDS. 2015. PMID: 26244394 Free PMC article.
-
Viruses and Human Milk: Transmission or Protection?Adv Nutr. 2023 Nov;14(6):1389-1415. doi: 10.1016/j.advnut.2023.08.007. Epub 2023 Aug 20. Adv Nutr. 2023. PMID: 37604306 Free PMC article. Review.
-
The role of neutralizing antibodies in prevention of HIV-1 infection: what can we learn from the mother-to-child transmission context?Retrovirology. 2013 Oct 7;10:103. doi: 10.1186/1742-4690-10-103. Retrovirology. 2013. PMID: 24099103 Free PMC article. Review.
-
Infant HIV type 1 gp120 vaccination elicits robust and durable anti-V1V2 immunoglobulin G responses and only rare envelope-specific immunoglobulin A responses.J Infect Dis. 2015 Feb 15;211(4):508-17. doi: 10.1093/infdis/jiu444. Epub 2014 Aug 27. J Infect Dis. 2015. PMID: 25170104 Free PMC article. Clinical Trial.
References
-
- WHO. PMTCT strategic vision 2010–2015: Preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals. Geneva, Switzerland: WHO Press; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

