Hepatoma-derived growth factor and nucleolin exist in the same ribonucleoprotein complex
- PMID: 23305559
- PMCID: PMC3551658
- DOI: 10.1186/1471-2091-14-2
Hepatoma-derived growth factor and nucleolin exist in the same ribonucleoprotein complex
Abstract
Background: Hepatoma-derived growth factor (HDGF) is a protein which is highly expressed in a variety of tumours. HDGF has mitogenic, angiogenic, neurotrophic and antiapoptotic activity but the molecular mechanisms by which it exerts these activities are largely unknown nor has its biological function in tumours been elucidated. Mass spectrometry was performed to analyse the HDGFStrep-tag interactome. By Pull-down-experiments using different protein and nucleic acid constructs the interaction of HDGF and nucleolin was investigated further.
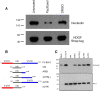
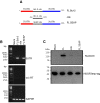
Results: A number of HDGFStrep-tag copurifying proteins were identified which interact with RNA or are involved in the cellular DNA repair machinery. The most abundant protein, however, copurifying with HDGF in this approach was nucleolin. Therefore we focus on the characterization of the interaction of HDGF and nucleolin in this study. We show that expression of a cytosolic variant of HDGF causes a redistribution of nucleolin into the cytoplasm. Furthermore, formation of HDGF/nucleolin complexes depends on bcl-2 mRNA. Overexpression of full length bcl-2 mRNA increases the number of HDGF/nucleolin complexes whereas expression of only the bcl-2 coding sequence abolishes interaction completely. Further examination reveals that the coding sequence of bcl-2 mRNA together with either the 5' or 3' UTR is sufficient for formation of HDGF/nucleolin complexes. When bcl-2 coding sequence within the full length cDNA is replaced by a sequence coding for secretory alkaline phosphatase complex formation is not enhanced.
Conclusion: The results provide evidence for the existence of HDGF and nucleolin containing nucleoprotein complexes which formation depends on the presence of specific mRNAs. The nature of these RNAs and other components of the complexes should be investigated in future.
Figures



References
-
- Nakamura H, Izumoto Y, Kambe H, Kuroda T, Mori T, Kawamura K, Yamamoto H, Kishimoto T. Molecular cloning of complementary DNA for a novel human hepatoma-derived growth factor. Its homology with high mobility group-1 protein. J Biol Chem. 1994;269(40):25143–25149. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

