Fibroblast growth factor 21 mediates specific glucagon actions
- PMID: 23305646
- PMCID: PMC3636653
- DOI: 10.2337/db12-1116
Fibroblast growth factor 21 mediates specific glucagon actions
Abstract
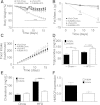
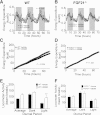
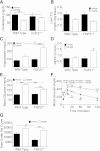
Glucagon, an essential regulator of glucose homeostasis, also modulates lipid metabolism and promotes weight loss, as reflected by the wasting observed in glucagonoma patients. Recently, coagonist peptides that include glucagon agonism have emerged as promising therapeutic candidates for the treatment of obesity and diabetes. We developed a novel stable and soluble glucagon receptor (GcgR) agonist, which allowed for in vivo dissection of glucagon action. As expected, chronic GcgR agonism in mice resulted in hyperglycemia and lower body fat and plasma cholesterol. Notably, GcgR activation also raised hepatic expression and circulating levels of fibroblast growth factor 21 (FGF21). This effect was retained in isolated primary hepatocytes from wild-type (WT) mice, but not GcgR knockout mice. We confirmed this link in healthy human volunteers, where injection of natural glucagon increased plasma FGF21 within hours. Functional relevance was evidenced in mice with genetic deletion of FGF21, where GcgR activation failed to induce the body weight loss and lipid metabolism changes observed in WT mice. Taken together, these data reveal for the first time that glucagon controls glucose, energy, and lipid metabolism at least in part via FGF21-dependent pathways.
Figures





Comment in
-
Role of fibroblast growth factor 21 in biology of glucagon.Diabetes. 2013 May;62(5):1376. doi: 10.2337/db12-1840. Diabetes. 2013. PMID: 23613555 Free PMC article. No abstract available.
References
-
- Gu W, Yan H, Winters KA, et al. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther 2009;331:871–881 - PubMed
-
- Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

