Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA
- PMID: 23305647
- PMCID: PMC3636623
- DOI: 10.2337/db12-0849
Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA
Abstract
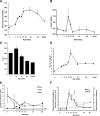
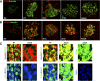
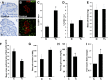
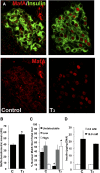
Neonatal β cells do not secrete glucose-responsive insulin and are considered immature. We previously showed the transcription factor MAFA is key for the functional maturation of β cells, but the physiological regulators of this process are unknown. Here we show that postnatal rat β cells express thyroid hormone (TH) receptor isoforms and deiodinases in an age-dependent pattern as glucose responsiveness develops. In vivo neonatal triiodothyronine supplementation and TH inhibition, respectively, accelerated and delayed metabolic development. In vitro exposure of immature islets to triiodothyronine enhanced the expression of Mafa, the secretion of glucose-responsive insulin, and the proportion of responsive cells, all of which are effects that were abolished in the presence of dominant-negative Mafa. Using chromatin immunoprecipitation and electrophoretic mobility shift assay, we show that TH has a direct receptor-ligand interaction with the Mafa promoter and, using a luciferase reporter, that this interaction was functional. Thus, TH can be considered a physiological regulator of functional maturation of β cells via its induction of Mafa.
Figures

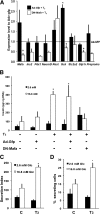

References
-
- Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 - PubMed
-
- D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–1401 - PubMed
-
- Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007;25:1940–1953 - PubMed
-
- Baetge EE. Production of beta-cells from human embryonic stem cells. Diabetes Obes Metab 2008;10(Suppl 4):186–194 - PubMed
-
- Bliss CR, Sharp GW. Glucose-induced insulin release in islets of young rats: time-dependent potentiation and effects of 2-bromostearate. Am J Physiol 1992;263:E890–E896 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK093909/DK/NIDDK NIH HHS/United States
- R01 DK 60127/DK/NIDDK NIH HHS/United States
- DK 076117/DK/NIDDK NIH HHS/United States
- R01 DK066056/DK/NIDDK NIH HHS/United States
- DK 36256/DK/NIDDK NIH HHS/United States
- R01 DK 66056/DK/NIDDK NIH HHS/United States
- R01 DK076117/DK/NIDDK NIH HHS/United States
- R01 DK060127/DK/NIDDK NIH HHS/United States
- P30 DK36836/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R01 DK036256/DK/NIDDK NIH HHS/United States
- DK 93909/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

