Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress
- PMID: 23306194
- PMCID: PMC3591590
- DOI: 10.1074/jbc.M112.445056
Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress
Abstract
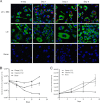
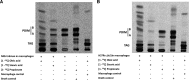
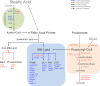
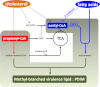
Recent data indicate that the nutrients available to Mycobacterium tuberculosis (Mtb) inside its host cell are restricted in their diversity. Fatty acids and cholesterol appear more favored; however, their degradation can result in certain metabolic stresses. Their breakdown can generate propionyl-CoA, which gives rise to potentially toxic intermediates. Detoxification of propionyl-CoA relies on the activity of the methylcitrate cycle, the methylmalonyl pathway, or incorporation of the propionyl-CoA into methyl-branched lipids in the cell wall. The current work explores carbon flux through these pathways, focusing primarily on those pathways responsible for the incorporation of propionyl-CoA into virulence-associated cell wall lipids. Exploiting both genetic and biochemical rescue, we demonstrate that these metabolic pressures are experienced by Mtb inside its host macrophage and that the bacterium accesses host fatty acid stores. The metabolism of these host lipids expands the acetyl-CoA pool and alleviates the pressure from propionyl-CoA. These data have major implications for our appreciation of central metabolism of Mtb during the course of infection.
Figures




References
-
- Cole S. T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S. V., Eiglmeier K., Gas S., Barry C. E., 3rd, Tekaia F., Badcock K., Basham D., Brown D., Chillingworth T., Connor R., Davies R., Devlin K., Feltwell T., Gentles S., Hamlin N., Holroyd S., Hornsby T., Jagels K., Krogh A., McLean J., Moule S., Murphy L., Oliver K., Osborne J., Quail M. A., Rajandream M. A., Rogers J., Rutter S., Seeger K., Skelton J., Squares R., Squares S., Sulston J. E., Taylor K., Whitehead S., Barrell B. G. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 - PubMed
-
- Liu K., Yu J., Russell D. G. (2003) pckA-deficient Mycobacterium bovis BCG shows attenuated virulence in mice and in macrophages. Microbiology 149, 1829–1835 - PubMed
-
- McKinney J. D., Höner zu Bentrup K., Muñoz-Elías E. J., Miczak A., Chen B., Chan W. T., Swenson D., Sacchettini J. C., Jacobs W. R., Jr., Russell D. G. (2000) Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735–738 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

