Epigenetic regulation of miR-17~92 contributes to the pathogenesis of pulmonary fibrosis
- PMID: 23306545
- PMCID: PMC3603596
- DOI: 10.1164/rccm.201205-0888OC
Epigenetic regulation of miR-17~92 contributes to the pathogenesis of pulmonary fibrosis
Abstract
Rationale: Idiopathic pulmonary fibrosis (IPF) is a disease of progressive lung fibrosis with a high mortality rate. In organ repair and remodeling, epigenetic events are important. MicroRNAs (miRNAs) regulate gene expression post-transcriptionally and can target epigenetic molecules important in DNA methylation. The miR-17~92 miRNA cluster is critical for lung development and lung epithelial cell homeostasis and is predicted to target fibrotic genes and DNA methyltransferase (DNMT)-1 expression.
Objectives: We investigated the miR-17~92 cluster expression and its role in regulating DNA methylation events in IPF lung tissue.
Methods: Expression and DNA methylation patterns of miR-17~92 were determined in human IPF lung tissue and fibroblasts and fibrotic mouse lung tissue. The relationship between the miR-17~92 cluster and DNMT-1 expression was examined in vitro. Using a murine model of pulmonary fibrosis, we examined the therapeutic potential of the demethylating agent, 5'-aza-2'-deoxycytidine.
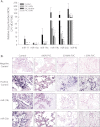
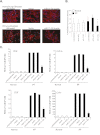
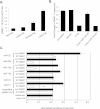
Measurements and main results: Compared with control samples, miR-17~92 expression was reduced in lung biopsies and lung fibroblasts from patients with IPF, whereas DNMT-1 expression and methylation of the miR-17~92 promoter was increased. Several miRNAs from the miR-17~92 cluster targeted DNMT-1 expression resulting in a negative feedback loop. Similarly, miR-17~92 expression was reduced in the lungs of bleomycin-treated mice. Treatment with 5'-aza-2'-deoxycytidine in a murine bleomycin-induced pulmonary fibrosis model reduced fibrotic gene and DNMT-1 expression, enhanced miR-17~92 cluster expression, and attenuated pulmonary fibrosis.
Conclusions: This study provides insight into the pathobiology of IPF and identifies a novel epigenetic feedback loop between miR-17~92 and DNMT-1 in lung fibrosis.
Figures



Comment in
-
The promise of epigenetic therapies in treatment of idiopathic pulmonary fibrosis.Am J Respir Crit Care Med. 2013 Feb 15;187(4):336-8. doi: 10.1164/rccm.201212-2272ED. Am J Respir Crit Care Med. 2013. PMID: 23418324 No abstract available.
-
Micro RNAs: the future of idiopathic pulmonary fibrosis therapy.Cell Biochem Biophys. 2015 Jan;71(1):509-11. doi: 10.1007/s12013-014-0217-9. Cell Biochem Biophys. 2015. PMID: 25164114 Free PMC article. No abstract available.
References
-
- Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431–440 - PubMed
-
- Magro CM, Waldman WJ, Knight DA, Allen JN, Nadasdy T, Frambach GE, Ross P, Marsh CB. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum Immunol 2006;67:284–297 - PubMed
-
- Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J 2007;30:835–839 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

