Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults
- PMID: 23306546
- PMCID: PMC3778736
- DOI: 10.1164/rccm.201208-1385OC
Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults
Abstract
Rationale: Tuberculosis (TB) is a major cause of morbidity and mortality worldwide, thus there is an urgent need for novel TB vaccines.
Objectives: We investigated a novel TB vaccine candidate, M72/AS01, in a phase IIa trial of bacille Calmette-Guérin-vaccinated, HIV-uninfected, and Mycobacterium tuberculosis (Mtb)-infected and -uninfected adults in South Africa.
Methods: Two doses of M72/AS01 were administered to healthy adults, with and without latent Mtb infection. Participants were monitored for 7 months after the first dose; cytokine production profiles, cell cycling, and regulatory phenotypes of vaccine-induced T cells were measured by flow cytometry.
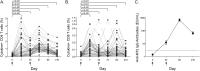
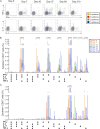
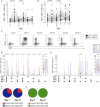
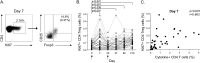
Measurements and main results: The vaccine had a clinically acceptable safety profile, and induced robust, long-lived M72-specific T-cell and antibody responses. M72-specific CD4 T cells produced multiple combinations of Th1 cytokines. Analysis of T-cell Ki67 expression showed that most vaccination-induced T cells did not express Th1 cytokines or IL-17; these cytokine-negative Ki67(+) T cells included subsets of CD4 T cells with regulatory phenotypes. PD-1, a negative regulator of activated T cells, was transiently expressed on M72-specific CD4 T cells after vaccination. Specific T-cell subsets were present at significantly higher frequencies after vaccination of Mtb-infected versus -uninfected participants.
Conclusions: M72/AS01 is clinically well tolerated in Mtb-infected and -uninfected adults, induces high frequencies of multifunctional T cells, and boosts distinct T-cell responses primed by natural Mtb infection. Moreover, these results provide important novel insights into how this immunity may be appropriately regulated after novel TB vaccination of Mtb-infected and -uninfected individuals.
Clinical trial registered with www.clinicaltrials.gov (NCT 00600782).
Figures

References
-
- World Health OrganizationGlobal tuberculosis control 2011 [accessed 2011 Dec 6]. Available from: http://www.who.int/tb/publications/global_report/en/index.html
-
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–1158. - PubMed
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA. 1994;271:698–702. - PubMed
-
- Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

