Novel mechanisms that pattern and shape the midbrain-hindbrain boundary
- PMID: 23307071
- PMCID: PMC11113640
- DOI: 10.1007/s00018-012-1240-x
Novel mechanisms that pattern and shape the midbrain-hindbrain boundary
Abstract
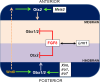
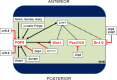
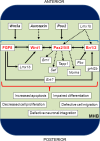
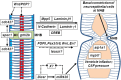
The midbrain-hindbrain boundary (MHB) is a highly conserved vertebrate signalling centre, acting to pattern and establish neural identities within the brain. While the core signalling pathways regulating MHB formation have been well defined, novel genetic and mechanistic processes that interact with these core components are being uncovered, helping to further elucidate the complicated networks governing MHB specification, patterning and shaping. Although formation of the MHB organiser is traditionally thought of as comprising three stages, namely positioning, induction and maintenance, we propose that a fourth stage, morphogenesis, should be considered as an additional stage in MHB formation. This review will examine evidence for novel factors regulating the first three stages of MHB development and will explore the evidence for regulation of MHB morphogenesis by non-classical MHB-patterning genes.
Figures
Similar articles
-
Gbx2 functions as a transcriptional repressor to regulate the specification and morphogenesis of the mid-hindbrain junction in a dosage- and stage-dependent manner.Mech Dev. 2013 Nov-Dec;130(11-12):532-52. doi: 10.1016/j.mod.2013.07.004. Epub 2013 Aug 7. Mech Dev. 2013. PMID: 23933069
-
Midbrain-hindbrain boundary patterning and morphogenesis are regulated by diverse grainy head-like 2-dependent pathways.Development. 2012 Feb;139(3):525-36. doi: 10.1242/dev.066522. Development. 2012. PMID: 22223680
-
Zebrafish gbx1 refines the midbrain-hindbrain boundary border and mediates the Wnt8 posteriorization signal.Neural Dev. 2009 Apr 2;4:12. doi: 10.1186/1749-8104-4-12. Neural Dev. 2009. PMID: 19341460 Free PMC article.
-
Fgf8 gene regulatory network and the isthmic organizer: an evolutionary perspective.Int J Dev Biol. 2024;68(4):211-222. doi: 10.1387/ijdb.240198ah. Int J Dev Biol. 2024. PMID: 40053062 Review.
-
Divide et Impera--the midbrain-hindbrain boundary and its organizer.Trends Neurosci. 2004 Dec;27(12):727-34. doi: 10.1016/j.tins.2004.10.003. Trends Neurosci. 2004. PMID: 15541513 Review.
Cited by
-
vox homeobox gene: a novel regulator of midbrain-hindbrain boundary development in medaka fish?Dev Genes Evol. 2016 Mar;226(2):99-107. doi: 10.1007/s00427-016-0533-8. Epub 2016 Mar 10. Dev Genes Evol. 2016. PMID: 26965282
-
Spatiotemporal transcriptomic maps of whole mouse embryos at the onset of organogenesis.Nat Genet. 2023 Jul;55(7):1176-1185. doi: 10.1038/s41588-023-01435-6. Epub 2023 Jul 6. Nat Genet. 2023. PMID: 37414952 Free PMC article.
-
Role of miRNA-9 in Brain Development.J Exp Neurosci. 2016 Oct 5;10:101-120. doi: 10.4137/JEN.S32843. eCollection 2016. J Exp Neurosci. 2016. PMID: 27721656 Free PMC article. Review.
-
Expression and function of microRNA-9 in the mid-hindbrain area of embryonic chick.BMC Dev Biol. 2018 Feb 22;18(1):3. doi: 10.1186/s12861-017-0159-8. BMC Dev Biol. 2018. PMID: 29471810 Free PMC article.
-
Molecular Determinants of A9 Dopaminergic Neurons.Neuromolecular Med. 2025 May 21;27(1):43. doi: 10.1007/s12017-025-08861-1. Neuromolecular Med. 2025. PMID: 40397062 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

