Clinical significance and novel mechanism of action of kallikrein 6 in glioblastoma
- PMID: 23307575
- PMCID: PMC3578488
- DOI: 10.1093/neuonc/nos313
Clinical significance and novel mechanism of action of kallikrein 6 in glioblastoma
Abstract
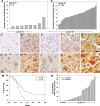
Background: Kallikreins have prognostic value in specific malignancies, but few studies have addressed their clinical significance to glioblastoma multiforme (GBM). Kallikrein 6 (KLK6) is of potential high relevance to GBM, since it is upregulated at sites of CNS pathology and linked to reactive astrogliosis. Here we examine the clinical value of KLK6 as a prognostic indicator of GBM patient survival and its activity in promoting resistance to cytotoxic agents.
Methods: The association between patient survival and levels of KLK6 immunoreactivity were investigated in 60 grade IV astrocytoma tumor specimens. Levels of KLK6 RNA were also evaluated in a separate set of GBM patient tumors (n = 23). Recombinant KLK6 or enforced KLK6 overexpression in GBM cell lines was used to evaluate effects on astrocytoma cell survival.
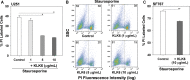
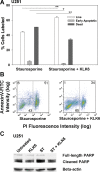
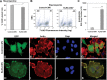
Results: A range of KLK6 expression was observed across grade IV tumors, with higher levels a poor prognostic indicator of patient survival (P = .02) even after adjusting for gender and Eastern Cooperative Oncology Group performance scores (P = .01). KLK6 reduced the sensitivity of GBM cell lines to cytotoxic agents, including staurosporine and cisplatin, and to the current standard of patient care: radiotherapy or temozolomide alone or in combination. The ability of KLK6 to promote resistance to apoptosis was dependent on activation of the thrombin receptor, protease activated receptor 1.
Conclusions: Taken together, these results indicate that elevated levels of KLK6 in GBM are likely to promote the resistance of tumor cells to cytotoxic agents and are an indicator of reduced patient postsurgical survival times.
Figures

Similar articles
-
Prognostic significance of multiple kallikreins in high-grade astrocytoma.BMC Cancer. 2015 Aug 1;15:565. doi: 10.1186/s12885-015-1566-5. BMC Cancer. 2015. PMID: 26231762 Free PMC article.
-
The DNA repair protein ALKBH2 mediates temozolomide resistance in human glioblastoma cells.Neuro Oncol. 2013 Mar;15(3):269-78. doi: 10.1093/neuonc/nos301. Epub 2012 Dec 20. Neuro Oncol. 2013. PMID: 23258843 Free PMC article.
-
Mitochondrial protein ATPase family, AAA domain containing 3A correlates with radioresistance in glioblastoma.Neuro Oncol. 2013 Oct;15(10):1342-52. doi: 10.1093/neuonc/not077. Neuro Oncol. 2013. PMID: 24057885 Free PMC article.
-
A Metabolic Inhibitory Cocktail for Grave Cancers: Metformin, Pioglitazone and Lithium Combination in Treatment of Pancreatic Cancer and Glioblastoma Multiforme.Biochem Genet. 2016 Oct;54(5):573-618. doi: 10.1007/s10528-016-9754-9. Epub 2016 Jul 4. Biochem Genet. 2016. PMID: 27377891 Review.
-
Analysis of Clinical Success and Molecular Mechanisms of Action of Novel Anti-glioblastoma Drugs: A Review.Curr Med Chem. 2025;32(6):1082-1102. doi: 10.2174/0109298673281283240101053940. Curr Med Chem. 2025. PMID: 38299393 Review.
Cited by
-
Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer.Oncotarget. 2016 Dec 20;7(51):85332-85348. doi: 10.18632/oncotarget.13352. Oncotarget. 2016. PMID: 27863404 Free PMC article.
-
Prognostic significance of multiple kallikreins in high-grade astrocytoma.BMC Cancer. 2015 Aug 1;15:565. doi: 10.1186/s12885-015-1566-5. BMC Cancer. 2015. PMID: 26231762 Free PMC article.
-
Kallikreins - The melting pot of activity and function.Biochimie. 2016 Mar;122:270-82. doi: 10.1016/j.biochi.2015.09.023. Epub 2015 Sep 25. Biochimie. 2016. PMID: 26408415 Free PMC article. Review.
-
Neuro-Immune Hemostasis: Homeostasis and Diseases in the Central Nervous System.Front Cell Neurosci. 2018 Nov 26;12:459. doi: 10.3389/fncel.2018.00459. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30534057 Free PMC article. Review.
-
The brain tissue response to surgical injury and its possible contribution to glioma recurrence.J Neurooncol. 2016 May;128(1):1-8. doi: 10.1007/s11060-016-2096-y. Epub 2016 Mar 9. J Neurooncol. 2016. PMID: 26961772 Review.
References
-
- Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18(8):1061–1083. - PubMed
-
- Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. - PubMed
-
- Gravendeel LA, Kouwenhoven MC, Gevaert O, et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009;69(23):9065–9072. - PubMed
-
- Lundwall A, Clauss A, Olsson AY. Evolution of kallikrein-related peptidases in mammals and identification of a genetic locus encoding potential regulatory inhibitors. Biol Chem. 2006;387(3):243–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

