Sp8 and COUP-TF1 reciprocally regulate patterning and Fgf signaling in cortical progenitors
- PMID: 23307639
- PMCID: PMC4014177
- DOI: 10.1093/cercor/bhs412
Sp8 and COUP-TF1 reciprocally regulate patterning and Fgf signaling in cortical progenitors
Abstract
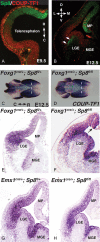
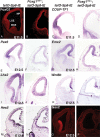
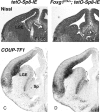
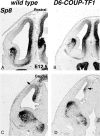
To gain new insights into the transcriptional regulation of cortical development, we examined the role of the transcription factor Sp8, which is downstream of Fgf8 signaling and known to promote rostral cortical development. We have used a binary transgenic system to express Sp8 throughout the mouse telencephalon in a temporally restricted manner. Our results show that misexpression of Sp8 throughout the telencephalon, at early but not late embryonic stages, results in cortical hypoplasia, which is accompanied by increased cell death, reduced proliferation, and precocious neuronal differentiation. Misexpression of Sp8 at early developmental stages represses COUP-TF1 expression, a negative effector of Fgf signaling and a key promoter of posterior cortical identity, while ablation of Sp8 has the opposite effect. In addition, transgenic misexpression of COUP-TF1 resulted in downregulation of Sp8, indicating a reciprocal cross-regulation between these 2 transcription factors. Although Sp8 has been suggested to induce and/or maintain Fgf8 expression in the embryonic telencephalon, neither Fgf8 nor Fgf15 was upregulated using our gain-of-function approach. However, misexpression of Sp8 greatly increased the expression of Fgf target molecules, suggesting enhanced Fgf signaling. Thus, we propose that Sp8 promotes rostral and dorsomedial cortical development by repressing COUP-TF1 and promoting Fgf signaling in pallial progenitors.
Keywords: Fgf signaling, neurogenesis; Sp8; corticogenesis; patterning; proliferation.
Figures

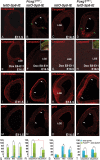
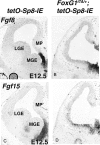
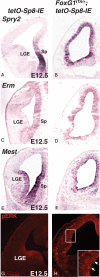
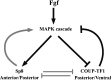
References
-
- Armentano M, Chou SJ, Srubek Tomassy G, Leingartner A, O'Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. - PubMed
-
- Bishop KM, Goudreau G, O'Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

