Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia
- PMID: 23307829
- PMCID: PMC3573704
- DOI: 10.1161/CIRCULATIONAHA.112.116103
Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia
Abstract
Background: Stem cells are thought to enhance vascular remodeling in ischemic tissue in part through paracrine effects. Using molecular imaging, we tested the hypothesis that treatment of limb ischemia with multipotential adult progenitor cells (MAPCs) promotes recovery of blood flow through the recruitment of proangiogenic monocytes.
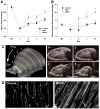
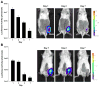
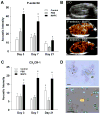
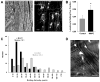
Methods and results: Hind-limb ischemia was produced in mice by iliac artery ligation, and MAPCs were administered intramuscularly on day 1. Optical imaging of luciferase-transfected MAPCs indicated that cells survived for 1 week. Contrast-enhanced ultrasound on days 3, 7, and 21 showed a more complete recovery of blood flow and greater expansion of microvascular blood volume in MAPC-treated mice than in controls. Fluorescent microangiography demonstrated more complete distribution of flow to microvascular units in MAPC-treated mice. On ultrasound molecular imaging, expression of endothelial P-selectin and intravascular recruitment of CX(3)CR-1-positive monocytes were significantly higher in MAPC-treated mice than in the control groups at days 3 and 7 after arterial ligation. Muscle immunohistology showed a >10-fold-greater infiltration of monocytes in MAPC-treated than control-treated ischemic limbs at all time points. Intravital microscopy of ischemic or tumor necrosis factor-α-treated cremaster muscle demonstrated that MAPCs migrate to perimicrovascular locations and potentiate selectin-dependent leukocyte rolling. In vitro migration of human CD14(+) monocytes was 10-fold greater in response to MAPC-conditioned than basal media.
Conclusions: In limb ischemia, MAPCs stimulate the recruitment of proangiogenic monocytes through endothelial activation and enhanced chemotaxis. These responses are sustained beyond the MAPC lifespan, suggesting that paracrine effects promote flow recovery by rebalancing the immune response toward a more regenerative phenotype.
Conflict of interest statement
Figures
References
-
- Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80:829–837. - PubMed
-
- Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking cc-chemokine receptor-2. Circ Res. 2004;94:671–677. - PubMed
-
- Milch HS, Schubert SY, Hammond S, Spiegel JH. Enhancement of ischemic wound healing by inducement of local angiogenesis. Laryngoscope. 2010;120:1744–1748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK063508/DK/NIDDK NIH HHS/United States
- R01 HL074443/HL/NHLBI NIH HHS/United States
- R01 HL111969/HL/NHLBI NIH HHS/United States
- RC1 HL100659/HL/NHLBI NIH HHS/United States
- R01 HL078610/HL/NHLBI NIH HHS/United States
- R01-DK-063508/DK/NIDDK NIH HHS/United States
- R01-HL-078610/HL/NHLBI NIH HHS/United States
- R01-HL-071141/HL/NHLBI NIH HHS/United States
- R01 HL071141/HL/NHLBI NIH HHS/United States
- RC1-HL-100659/HL/NHLBI NIH HHS/United States
- T32 HL094294/HL/NHLBI NIH HHS/United States
- T32-HL094294/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

