Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance
- PMID: 23307858
- PMCID: PMC3618605
- DOI: 10.1158/1078-0432.CCR-12-1281
Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance
Abstract
Purpose: To identify mediators of glioblastoma antiangiogenic therapy resistance and target these mediators in xenografts.
Experimental design: We conducted microarray analysis comparing bevacizumab-resistant glioblastomas (BRG) with pretreatment tumors from the same patients. We established novel xenograft models of antiangiogenic therapy resistance to target candidate resistance mediator(s).
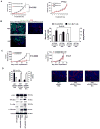
Results: BRG microarray analysis revealed upregulation versus pretreatment of receptor tyrosine kinase c-Met, which underwent further investigation because of its prior biologic plausibility as a bevacizumab resistance mediator. BRGs exhibited increased hypoxia versus pretreatment in a manner correlating with their c-Met upregulation, increased c-Met phosphorylation, and increased phosphorylation of c-Met-activated focal adhesion kinase and STAT3. We developed 2 novel xenograft models of antiangiogenic therapy resistance. In the first model, serial bevacizumab treatment of an initially responsive xenograft generated a xenograft with acquired bevacizumab resistance, which exhibited upregulated c-Met expression versus pretreatment. In the second model, a BRG-derived xenograft maintained refractoriness to the MRI tumor vasculature alterations and survival-promoting effects of bevacizumab. Growth of this BRG-derived xenograft was inhibited by a c-Met inhibitor. Transducing these xenograft cells with c-Met short hairpin RNA inhibited their invasion and survival in hypoxia, disrupted their mesenchymal morphology, and converted them from bevacizumab-resistant to bevacizumab-responsive. Engineering bevacizumab-responsive cells to express constitutively active c-Met caused these cells to form bevacizumab-resistant xenografts.
Conclusion: These findings support the role of c-Met in survival in hypoxia and invasion, features associated with antiangiogenic therapy resistance, and growth and therapeutic resistance of xenografts resistant to antiangiogenic therapy. Therapeutically targeting c-Met could prevent or overcome antiangiogenic therapy resistance.
©2012 AACR.
Conflict of interest statement
Conflicts of interest: W.S.C. is co-Founder, CEO, and shareholder of OncoSynergy. M.K.A. is a Scientific Advisory Board Member and shareholder of OncoSynergy.
Figures





Comment in
-
Glioblastoma resistance to anti-VEGF therapy: has the challenge been MET?Clin Cancer Res. 2013 Apr 1;19(7):1631-3. doi: 10.1158/1078-0432.CCR-13-0051. Epub 2013 Feb 12. Clin Cancer Res. 2013. PMID: 23403631 Free PMC article.
References
-
- Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. - PubMed
-
- Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. - PubMed
-
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. - PubMed
-
- Clark AJ, Lamborn KR, Butowski NA, Chang SM, Prados MD, Clarke JL, et al. Neurosurgical management and prognosis of patients with glioblastoma that progress during bevacizumab treatment. Neurosurgery. 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

