Mouse TU tagging: a chemical/genetic intersectional method for purifying cell type-specific nascent RNA
- PMID: 23307870
- PMCID: PMC3553287
- DOI: 10.1101/gad.205278.112
Mouse TU tagging: a chemical/genetic intersectional method for purifying cell type-specific nascent RNA
Erratum in
- Genes Dev. 2014 Dec 1;28(23):2677
Abstract
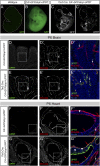
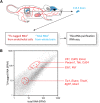
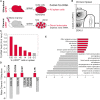
Transcriptional profiling is a powerful approach for understanding development and disease. Current cell type-specific RNA purification methods have limitations, including cell dissociation trauma or inability to identify all RNA species. Here, we describe "mouse thiouracil (TU) tagging," a genetic and chemical intersectional method for covalent labeling and purification of cell type-specific RNA in vivo. Cre-induced expression of uracil phosphoribosyltransferase (UPRT) provides spatial specificity; injection of 4-thiouracil (4TU) provides temporal specificity. Only UPRT(+) cells exposed to 4TU produce thio-RNA, which is then purified for RNA sequencing (RNA-seq). This method can purify transcripts from spatially complex and rare (<5%) cells, such as Tie2:Cre(+) brain endothelia/microglia (76% validated by expression pattern), or temporally dynamic transcripts, such as those acutely induced by lipopolysaccharide (LPS) injection. Moreover, generating chimeric mice via UPRT(+) bone marrow transplants identifies immune versus niche spleen RNA. TU tagging provides a novel method for identifying actively transcribed genes in specific cells at specific times within intact mice.
Figures




Similar articles
-
TU-Tagging: A Method for Identifying Layer-Enriched Neuronal Genes in Developing Mouse Visual Cortex.eNeuro. 2017 Oct 4;4(5):ENEURO.0181-17.2017. doi: 10.1523/ENEURO.0181-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 29085897 Free PMC article.
-
Applying thiouracil tagging to mouse transcriptome analysis.Nat Protoc. 2014 Feb;9(2):410-20. doi: 10.1038/nprot.2014.023. Epub 2014 Jan 23. Nat Protoc. 2014. PMID: 24457332 Free PMC article.
-
Identification of sensory hair-cell transcripts by thiouracil-tagging in zebrafish.BMC Genomics. 2015 Oct 23;16:842. doi: 10.1186/s12864-015-2072-5. BMC Genomics. 2015. PMID: 26494580 Free PMC article.
-
Uncovering cell type-specific complexities of gene expression and RNA metabolism by TU-tagging and EC-tagging.Wiley Interdiscip Rev Dev Biol. 2018 Jul;7(4):e315. doi: 10.1002/wdev.315. Epub 2018 Jan 25. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29369522 Review.
-
Global analysis of RNA metabolism using bio-orthogonal labeling coupled with next-generation RNA sequencing.Methods. 2019 Feb 15;155:88-103. doi: 10.1016/j.ymeth.2018.12.001. Epub 2018 Dec 6. Methods. 2019. PMID: 30529548 Free PMC article. Review.
Cited by
-
FoxA1 and FoxA2 drive gastric differentiation and suppress squamous identity in NKX2-1-negative lung cancer.Elife. 2018 Nov 26;7:e38579. doi: 10.7554/eLife.38579. Elife. 2018. PMID: 30475207 Free PMC article.
-
The LepR-mediated leptin transport across brain barriers controls food reward.Mol Metab. 2018 Feb;8:13-22. doi: 10.1016/j.molmet.2017.12.001. Epub 2017 Dec 7. Mol Metab. 2018. PMID: 29254602 Free PMC article.
-
GIMAP6 regulates autophagy, immune competence, and inflammation in mice and humans.J Exp Med. 2022 Jun 6;219(6):e20201405. doi: 10.1084/jem.20201405. Epub 2022 May 13. J Exp Med. 2022. PMID: 35551368 Free PMC article.
-
TU-Tagging: A Method for Identifying Layer-Enriched Neuronal Genes in Developing Mouse Visual Cortex.eNeuro. 2017 Oct 4;4(5):ENEURO.0181-17.2017. doi: 10.1523/ENEURO.0181-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 29085897 Free PMC article.
-
Selective 4-Thiouracil Labeling of RNA Transcripts within Latently Infected Cells after Infection with Human Cytomegalovirus Expressing Functional Uracil Phosphoribosyltransferase.J Virol. 2018 Oct 12;92(21):e00880-18. doi: 10.1128/JVI.00880-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30089702 Free PMC article.
References
-
- Balda MS, Matter K 2009. Tight junctions and the regulation of gene expression. Biochim Biophys Acta 1788: 761–767 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
