Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults
- PMID: 23307876
- PMCID: PMC3613948
- DOI: 10.2215/CJN.04760512
Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults
Abstract
Background and objectives: Atypical hemolytic uremic syndrome (aHUS) is a rare complement-mediated kidney disease that was first recognized in children but also affects adults. This study assessed the disease presentation and outcome in a nationwide cohort of patients with aHUS according to the age at onset and the underlying complement abnormalities.
Design, setting, participants, & measurements: A total of 214 patients with aHUS were enrolled between 2000 and 2008 and screened for mutations in the six susceptibility factors for aHUS and for anti-factor H antibodies.
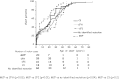
Results: Onset of aHUS occurred as frequently during adulthood (58.4%) as during childhood (41.6%). The percentages of patients who developed the disease were 23%, 40%, 70%, and 98% by age 2, 18, 40, and 60 years, respectively. Mortality was higher in children than in adults (6.7% versus 0.8% at 1 year) (P=0.02), but progression to ESRD after the first aHUS episode was more frequent in adults (46% versus 16%; P<0.001). Sixty-one percent of patients had mutations in their complement genes. The renal outcome was not significantly different in adults regardless of genetic background. Only membrane cofactor protein (MCP) and undetermined aHUS were less severe in children than adults. The frequency of relapse after 1 year was 92% in children with MCP-associated HUS and approximately 30% in all other subgroups.
Conclusion: Mortality rate was higher in children than adults with aHUS, but renal prognosis was worse in adults than children. In children, the prognosis strongly depends on the genetic background.
Figures



References
-
- Noris M, Remuzzi G: Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 2009 - PubMed
-
- Dragon-Durey MA, Sethi SK, Bagga A, Blanc C, Blouin J, Ranchin B, André JL, Takagi N, Cheong HI, Hari P, Le Quintrec M, Niaudet P, Loirat C, Fridman WH, Frémeaux-Bacchi V: Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol 21: 2180–2187, 2010 - PMC - PubMed
-
- Roumenina LT, Jablonski M, Hue C, Blouin J, Dimitrov JD, Dragon-Durey MA, Cayla M, Fridman WH, Macher MA, Ribes D, Moulonguet L, Rostaing L, Satchell SC, Mathieson PW, Sautes-Fridman C, Loirat C, Regnier CH, Halbwachs-Mecarelli L, Fremeaux-Bacchi V: Hyperfunctional C3 convertase leads to complement deposition on endothelial cells and contributes to atypical hemolytic uremic syndrome. Blood 114: 2837–2845, 2009 - PubMed
-
- Roumenina LT, Frimat M, Miller EC, Provot F, Dragon-Durey MA, Bordereau P, Bigot S, Hue C, Satchell SC, Mathieson PW, Mousson C, Noel C, Sautes-Fridman C, Halbwachs-Mecarelli L, Atkinson JP, Lionet A, Fremeaux-Bacchi V: A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood 119: 4182–4191, 2012 - PMC - PubMed
-
- Fremeaux-Bacchi V, Kemp EJ, Goodship JA, Dragon-Durey MA, Strain L, Loirat C, Deng HW, Goodship TH: The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: Evidence from two independent cohorts. J Med Genet 42: 852–856, 2005 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

