Alterations of retinal vasculature in cystathionine-Beta-synthase mutant mice, a model of hyperhomocysteinemia
- PMID: 23307965
- PMCID: PMC3564451
- DOI: 10.1167/iovs.12-10536
Alterations of retinal vasculature in cystathionine-Beta-synthase mutant mice, a model of hyperhomocysteinemia
Abstract
Purpose: Mice with moderate/severe hyperhomocysteinemia due to deficiency or absence of the cbs gene encoding cystathionine-beta-synthase (CBS) have marked retinal disruption, ganglion cell loss, optic nerve mitochondrial dysfunction, and ERG defects; those with mild hyperhomocysteinemia have delayed retinal morphological/functional phenotype. Excess homocysteine is a risk factor for cardiovascular diseases; however, it is not known whether excess homocysteine alters retinal vasculature.
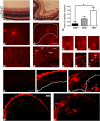
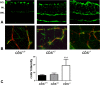
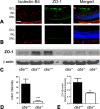
Methods: Cbs(+/+), cbs(+/-), and cbs(-/-) mice (age ∼3 weeks) were subjected to angiography; retinas were harvested for cryosections, flat-mount preparations, or trypsin digestion and subjected to immunofluorescence microscopy to visualize vessels using isolectin-B4, to detect angiogenesis using anti-VEGF and anti-endoglin (anti-CD105) and activated glial cells (anti-glial fibrillary acidic protein [anti-GFAP]) and to investigate the blood-retinal barrier using the tight junction markers zonula occludens-1 (ZO-1) and occludin. Expression of vegf was determined by quantitative RT-PCR (qRT-PCR) and immunoblotting. Human retinal endothelial cells (HRECs) were treated with excess homocysteine to analyze permeability.
Results: Angiography revealed vascular leakage in cbs(-/-) mice; immunohistochemical analysis demonstrated vascular patterns consistent with ischemia; isolectin-B4 labeling revealed a capillary-free zone centrally and new vessels with capillary tufts midperipherally. This was associated with increased vegf mRNA and protein, CD105, and GFAP in cbs(-/-) retinas concomitant with a marked decrease in ZO-1 and occludin. Homocysteine-treated HRECs showed increased permeability.
Conclusions: Severe elevation of homocysteine in cbs(-/-) mutant mice is accompanied by alterations in retinal vasculature (ischemia, neovascularization, and incompetent blood-retinal barrier). The marked disruption of retinal structure and decreased visual function reported in cbs(-/-) mice may reflect vasculopathy as well as neuropathy.
Conflict of interest statement
Disclosure:
Figures




References
-
- Perła-Kaján J, Twardowski T, Jakubowski H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 2007; 32: 561–572 - PubMed
-
- Mudd SH. Hypermethioninemias of genetic and non-genetic origin: a review. Am J Med Genet C Semin Med Genet. 2011; 157: 3–32 - PubMed
-
- Bleich S, Jünemann A, Von Ahsen N, et al. Homocysteine and risk of open-angle glaucoma. J Neural Transm. 2002; 109: 1499–1504 - PubMed
-
- Vessani RM, Ritch R, Liebmann JM, Jofe M. Plasma homocysteine is elevated in patients with exfoliation syndrome. Am J Ophthalmol. 2003; 136: 41–46 - PubMed
-
- Jaksic V, Markovic V, Milenkovic S, Stefanovic I, Jakovic N, Knezevic M. MTHFR. C677T homozygous mutation in a patient with pigmentary glaucoma and central retinal vein occlusion. Ophthalmic Res. 2010; 43: 193–196 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

