E-cadherin controls bronchiolar progenitor cells and onset of preneoplastic lesions in mice
- PMID: 23308049
- PMCID: PMC3540942
- DOI: 10.1593/neo.121088
E-cadherin controls bronchiolar progenitor cells and onset of preneoplastic lesions in mice
Abstract
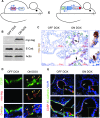
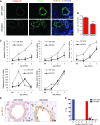
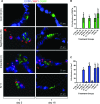
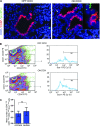
Although progenitor cells of the conducting airway have been spatially localized and some insights have been gained regarding their molecular phenotype, relatively little is known about the mechanisms regulating their maintenance, activation, and differentiation. This study investigates the potential roles of E-cadherin in mouse Clara cells, as these cells were shown to represent the progenitor/stem cells of the conducting airways and have been implicated as the cell of origin of human non-small cell lung cancer. Postnatal inactivation of E-cadherin affected Clara cell differentiation and compromised airway regeneration under injury conditions. In steady-state adult lung, overexpression of the dominant negative E-cadherin led to an expansion of the bronchiolar stem cells and decreased differentiation concomitant with canonical Wnt signaling activation. Expansion of the bronchiolar stem cell pool was associated with an incessant proliferation of neuroepithelial body.associated Clara cells that ultimately gave rise to bronchiolar hyperplasia. Despite progressive hyperplasia, only a minority of the mice developed pulmonary solid tumors, suggesting that the loss of E-cadherin function leads to tumor formation when additional mutations are sustained. The present study reveals that E-cadherin plays a critical role in the regulation of proliferation and homeostasis of the epithelial cells lining the conducting airways.
Figures



Similar articles
-
beta-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium.Am J Respir Cell Mol Biol. 2009 Nov;41(5):535-43. doi: 10.1165/rcmb.2008-0407OC. Epub 2009 Feb 12. Am J Respir Cell Mol Biol. 2009. PMID: 19213872 Free PMC article.
-
Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis.Am J Respir Crit Care Med. 2011 Feb 15;183(4):511-21. doi: 10.1164/rccm.201005-0744OC. Epub 2010 Sep 24. Am J Respir Crit Care Med. 2011. PMID: 20870756 Free PMC article.
-
ABL kinase inhibition promotes lung regeneration through expansion of an SCGB1A1+ SPC+ cell population following bacterial pneumonia.Proc Natl Acad Sci U S A. 2019 Jan 29;116(5):1603-1612. doi: 10.1073/pnas.1816030116. Epub 2019 Jan 17. Proc Natl Acad Sci U S A. 2019. PMID: 30655340 Free PMC article.
-
Clara cell: progenitor for the bronchiolar epithelium.Int J Biochem Cell Biol. 2010 Jan;42(1):1-4. doi: 10.1016/j.biocel.2009.09.002. Epub 2009 Sep 9. Int J Biochem Cell Biol. 2010. PMID: 19747565 Free PMC article. Review.
-
The multiple facets of the club cell in the pulmonary epithelium.Histol Histopathol. 2024 Aug;39(8):969-982. doi: 10.14670/HH-18-713. Epub 2024 Jan 18. Histol Histopathol. 2024. PMID: 38329181 Review.
Cited by
-
The clinicopathological significance and potential drug target of E-cadherin in NSCLC.Tumour Biol. 2015 Aug;36(8):6139-48. doi: 10.1007/s13277-015-3298-1. Epub 2015 Mar 11. Tumour Biol. 2015. PMID: 25758052
-
Fungal Aeroallergens-The Impact of Climate Change.J Fungi (Basel). 2023 May 7;9(5):544. doi: 10.3390/jof9050544. J Fungi (Basel). 2023. PMID: 37233255 Free PMC article. Review.
-
Overcoming intratumor heterogeneity of polygenic cancer drug resistance with improved biomarker integration.Neoplasia. 2012 Dec;14(12):1278-89. doi: 10.1593/neo.122096. Neoplasia. 2012. PMID: 23308059 Free PMC article.
-
ITGB4 deficiency in bronchial epithelial cells directs airway inflammation and bipolar disorder-related behavior.J Neuroinflammation. 2018 Aug 31;15(1):246. doi: 10.1186/s12974-018-1283-5. J Neuroinflammation. 2018. PMID: 30170608 Free PMC article.
-
Epithelial cell dysfunction, a major driver of asthma development.Allergy. 2020 Aug;75(8):1902-1917. doi: 10.1111/all.14421. Epub 2020 Jun 16. Allergy. 2020. PMID: 32460363 Free PMC article. Review.
References
-
- Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. - PubMed
-
- Blenkinsopp WK. Proliferation of respiratory tract epithelium in the rat. Exp Cell Res. 1967;46:144–154. - PubMed
-
- Evans MJ, Cabral LC, Stephens RJ, Freeman G. Acute kinetic response and renewal of the alveolar epithelium following injury by nitrogen dioxide. Chest. 1974;65(suppl):62S–65S. - PubMed
-
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. - PubMed
Supplementary References
-
- Dahl U, Sjodin A, Semb H. Cadherins regulate aggregation of pancreatic β-cells in vivo. Development. 1996;122:2895–2902. - PubMed
-
- Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem. 2002;277:4519–4525. - PubMed
-
- Van Winkle LS, Gunderson AD, Shimizu JA, Baker GL, Brown CD. Gender differences in naphthalene metabolism and naphthalene-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1122–L1134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
