4'-Phosphopantetheinyl transferase PptT, a new drug target required for Mycobacterium tuberculosis growth and persistence in vivo
- PMID: 23308068
- PMCID: PMC3534377
- DOI: 10.1371/journal.ppat.1003097
4'-Phosphopantetheinyl transferase PptT, a new drug target required for Mycobacterium tuberculosis growth and persistence in vivo
Abstract
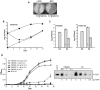
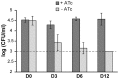
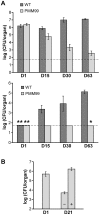
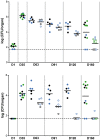
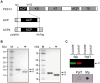
The cell envelope of Mycobacterium tuberculosis, the causative agent of tuberculosis in humans, contains lipids with unusual structures. These lipids play a key role in both virulence and resistance to the various hostile environments encountered by the bacteria during infection. They are synthesized by complex enzymatic systems, including type-I polyketide synthases and type-I and -II fatty acid synthases, which require a post-translational modification to become active. This modification consists of the covalent attachment of the 4'-phosphopantetheine moiety of Coenzyme A catalyzed by phosphopantetheinyl transferases (PPTases). PptT, one of the two PPTases produced by mycobacteria, is involved in post-translational modification of various type-I polyketide synthases required for the formation of both mycolic acids and lipid virulence factors in mycobacteria. Here we identify PptT as a new target for anti-tuberculosis drugs; we address all the critical issues of target validation to demonstrate that PptT can be used to search for new drugs. We confirm that PptT is essential for the growth of M. bovis BCG in vitro and show that it is required for persistence of M. bovis BCG in both infected macrophages and immunodeficient mice. We generated a conditional expression mutant of M. tuberculosis, in which the expression of the pptT gene is tightly regulated by tetracycline derivatives. We used this construct to demonstrate that PptT is required for the replication and survival of the tubercle bacillus during the acute and chronic phases of infection in mice. Finally, we developed a robust and miniaturized assay based on scintillation proximity assay technology to search for inhibitors of PPTases, and especially of PptT, by high-throughput screening. Our various findings indicate that PptT meets the key criteria for being a therapeutic target for the treatment of mycobacterial infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
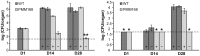

Similar articles
-
Structure, biochemistry, and inhibition of essential 4'-phosphopantetheinyl transferases from two species of Mycobacteria.ACS Chem Biol. 2014 Sep 19;9(9):1939-44. doi: 10.1021/cb500263p. Epub 2014 Jul 9. ACS Chem Biol. 2014. PMID: 24963544 Free PMC article.
-
AcpM, the meromycolate extension acyl carrier protein of Mycobacterium tuberculosis, is activated by the 4'-phosphopantetheinyl transferase PptT, a potential target of the multistep mycolic acid biosynthesis.Biochemistry. 2015 Apr 14;54(14):2360-71. doi: 10.1021/bi501444e. Epub 2015 Apr 1. Biochemistry. 2015. PMID: 25785780
-
The nonredundant roles of two 4'-phosphopantetheinyl transferases in vital processes of Mycobacteria.Proc Natl Acad Sci U S A. 2006 May 30;103(22):8511-6. doi: 10.1073/pnas.0511129103. Epub 2006 May 18. Proc Natl Acad Sci U S A. 2006. PMID: 16709676 Free PMC article.
-
Targeting polyketide synthase 13 for the treatment of tuberculosis.Eur J Med Chem. 2023 Nov 5;259:115702. doi: 10.1016/j.ejmech.2023.115702. Epub 2023 Aug 1. Eur J Med Chem. 2023. PMID: 37544185 Review.
-
Mycolic acids: deciphering and targeting the Achilles' heel of the tubercle bacillus.Mol Microbiol. 2015 Oct;98(1):7-16. doi: 10.1111/mmi.13101. Epub 2015 Jul 30. Mol Microbiol. 2015. PMID: 26135034 Free PMC article. Review.
Cited by
-
Insights from the docking and molecular dynamics simulation of the Phosphopantetheinyl transferase (PptT) structural model from Mycobacterium tuberculosis.Bioinformation. 2013 Jul 17;9(13):685-9. doi: 10.6026/97320630009685. Print 2013. Bioinformation. 2013. PMID: 23930020 Free PMC article.
-
Antifungal Drugs.Metabolites. 2020 Mar 12;10(3):106. doi: 10.3390/metabo10030106. Metabolites. 2020. PMID: 32178468 Free PMC article. Review.
-
Structure, biochemistry, and inhibition of essential 4'-phosphopantetheinyl transferases from two species of Mycobacteria.ACS Chem Biol. 2014 Sep 19;9(9):1939-44. doi: 10.1021/cb500263p. Epub 2014 Jul 9. ACS Chem Biol. 2014. PMID: 24963544 Free PMC article.
-
Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform.Nat Microbiol. 2017 Feb 6;2:16274. doi: 10.1038/nmicrobiol.2016.274. Nat Microbiol. 2017. PMID: 28165460 Free PMC article.
-
Opposing reactions in coenzyme A metabolism sensitize Mycobacterium tuberculosis to enzyme inhibition.Science. 2019 Feb 1;363(6426):eaau8959. doi: 10.1126/science.aau8959. Science. 2019. PMID: 30705156 Free PMC article.
References
-
- Almeida Da Silva PE, Palomino JC (2011) Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemoth 66: 1417–1430. - PubMed
-
- Pieters J (2008) Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe 3: 399–407. - PubMed
-
- Stewart GR, Robertson BD, Young DB (2003) Tuberculosis: a problem with persistence. Nat Rev Microbiol 1: 97–105. - PubMed
-
- Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K (2002) Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43: 717–731. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

