Structure-function relation of phospholamban: modulation of channel activity as a potential regulator of SERCA activity
- PMID: 23308118
- PMCID: PMC3537670
- DOI: 10.1371/journal.pone.0052744
Structure-function relation of phospholamban: modulation of channel activity as a potential regulator of SERCA activity
Abstract
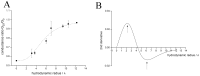
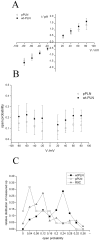
Phospholamban (PLN) is a small integral membrane protein, which binds and inhibits in a yet unknown fashion the Ca(2+)-ATPase (SERCA) in the sarcoplasmic reticulum. When reconstituted in planar lipid bilayers PLN exhibits ion channel activity with a low unitary conductance. From the effect of non-electrolyte polymers on this unitary conductance we estimate a narrow pore with a diameter of ca. 2.2 Å for this channel. This value is similar to that reported for the central pore in the structure of the PLN pentamer. Hence the PLN pentamer, which is in equilibrium with the monomer, is the most likely channel forming structure. Reconstituted PLN mutants, which either stabilize (K27A and R9C) or destabilize (I47A) the PLN pentamer and also phosphorylated PLN still generate the same unitary conductance of the wt/non-phosphorylated PLN. However the open probability of the phosphorylated PLN and of the R9C mutant is significantly lower than that of the respective wt/non-phosphorylated control. In the context of data on PLN/SERCA interaction and on Ca(2+) accumulation in the sarcoplasmic reticulum the present results are consistent with the view that PLN channel activity could participate in the balancing of charge during Ca(2+) uptake. A reduced total conductance of the K(+) transporting PLN by phosphorylation or by the R9C mutation may stimulate Ca(2+) uptake in the same way as an inhibition of K(+) channels in the SR membrane. The R9C-PLN mutation, a putative cause of dilated cardiomyopathy, might hence affect SERCA activity also via its inherent low open probability.
Conflict of interest statement
Figures



References
-
- Schmidt AG, Edes I, Kranias EG (2001) Phospholamban: a promising therapeutic target in heart failure? Cardiovasc Drugs Ther 15: 387–396. - PubMed
-
- Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Guidelli R, Inesi G (2006) Pre-steady State Electrogenic Events of Ca2+/H+ Exchange and Transport by the Ca2+-ATPase. J Biol Chem 281: 37720–37727. - PubMed
-
- Cornea RL, Jones LR, Autry JM, Thomas DD (1997) Mutation and Phosphorylation Change the Oligomeric Structure of Phospholamban in Lipid Bilayers. Biochemistry 36: 2960–2967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

