Inhibitory influence of Enterococcus faecium on the propagation of swine influenza A virus in vitro
- PMID: 23308134
- PMCID: PMC3538747
- DOI: 10.1371/journal.pone.0053043
Inhibitory influence of Enterococcus faecium on the propagation of swine influenza A virus in vitro
Abstract
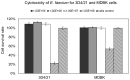
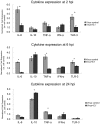
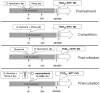
The control of infectious diseases such as swine influenza viruses (SwIV) plays an important role in food production both from the animal health and from the public health point of view. Probiotic microorganisms and other health improving food supplements have been given increasing attention in recent years, but, no information on the effects of probiotics on swine influenza virus is available. Here we address this question by assessing the inhibitory potential of the probiotic Enterococcus faecium NCIMB 10415 (E. faecium) on the replication of two porcine strains of influenza virus (H1N1 and H3N2 strain) in a continuous porcine macrophage cell line (3D4/21) and in MDBK cells. Cell cultures were treated with E. faecium at the non-toxic concentration of 1×10(6) CFU/ml in growth medium for 60 to 90 min before, during and after SwIV infection. After further incubation of cultures in probiotic-free growth medium, cell viability and virus propagation were determined at 48 h or 96 h post infection. The results obtained reveal an almost complete recovery of viability of SwIV infected cells and an inhibition of virus multiplication by up to four log units in the E. faecium treated cells. In both 3D4/21- and MDBK-cells a 60 min treatment with E. faecium stimulated nitric oxide (NO) release which is in line with published evidence for an antiviral function of NO. Furthermore, E. faecium caused a modified cellular expression of selected mediators of defence in 3D4-cells: while the expression of TNF-α, TLR-3 and IL-6 were decreased in the SwIV-infected and probiotic treated cells, IL-10 was found to be increased. Since we obtained experimental evidence for the direct adsorptive trapping of SwIV through E. faecium, this probiotic microorganism inhibits influenza viruses by at least two mechanisms, direct physical interaction and strengthening of innate defence at the cellular level.
Conflict of interest statement
Figures


References
-
- Fineberg HV (2008) Preparing for avian influenza: lessons from the “swine flu affair”. J Infect Dis 197 (Suppl 1) S14–18. - PubMed
-
- Yasui H, Shida K, Matsuzaki T, Yokokura T (1999) Immunomodulatory function of lactic acid bacteria. Antonie Van Leeuwenhoek 76: 383–389. - PubMed
-
- Clancy R (2003) Immunobiotics and the probiotic evolution. FEMS Immunol Med Microbiol 38: 9–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

