Growth associated protein 43 is expressed in skeletal muscle fibers and is localized in proximity of mitochondria and calcium release units
- PMID: 23308181
- PMCID: PMC3538766
- DOI: 10.1371/journal.pone.0053267
Growth associated protein 43 is expressed in skeletal muscle fibers and is localized in proximity of mitochondria and calcium release units
Abstract
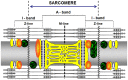
The neuronal Growth Associated Protein 43 (GAP43), also known as B-50 or neuromodulin, is involved in mechanisms controlling pathfinding and branching of neurons during development and regeneration. For many years this protein was classified as neuron-specific, but recent evidences suggest that a) GAP43 is expressed in the nervous system not only in neurons, but also in glial cells, and b) probably it is present also in other tissues. In particular, its expression was revealed in muscles from patients affected by various myopathies, indicating that GAP43 can no-longer considered only as a neuron-specific molecule. We have investigated the expression and subcellular localization of GAP43 in mouse satellite cells, myotubes, and adult muscle (extensor digitorum longus or EDL) using Western blotting, immuno-fluorescence combined to confocal microscopy and electron microscopy. Our in vitro results indicated that GAP43 is indeed expressed in both myoblasts and differentiating myotubes, and its cellular localization changes dramatically during maturation: in myoblasts the localization appeared to be mostly nuclear, whereas with differentiation the protein started to display a sarcomeric-like pattern. In adult fibers, GAP43 expression was evident with the protein labeling forming (in longitudinal views) a double cross striation reminiscent of the staining pattern of other organelles, such as calcium release units (CRUs) and mitochondria. Double immuno-staining and experiments done in EDL muscles fixed at different sarcomere lengths, allowed us to determine the localization, from the sarcomere Z-line, of GAP43 positive foci, falling between that of CRUs and of mitochondria. Staining of cross sections added a detail to the puzzle: GAP43 labeling formed a reticular pattern surrounding individual myofibrils, but excluding contractile elements. This work leads the way to further investigation about the possible physiological and structural role of GAP43 protein in adult fiber function and disease.
Conflict of interest statement
Figures






Similar articles
-
Specific association of growth-associated protein 43 with calcium release units in skeletal muscles of lower vertebrates.Eur J Histochem. 2014 Dec 5;58(4):2453. doi: 10.4081/ejh.2014.2453. Eur J Histochem. 2014. PMID: 25578978 Free PMC article.
-
Mitochondria Association to Calcium Release Units is Controlled by Age and Muscle Activity.Eur J Transl Myol. 2015 Oct 27;25(4):257-62. doi: 10.4081/ejtm.2015.5604. eCollection 2015 Aug 24. Eur J Transl Myol. 2015. PMID: 26913166 Free PMC article.
-
Discrete localization patterns of Arf6, and its activators EFA6A and BRAG2, and its effector PIP5kinaseγ on myofibrils of myotubes and plasma membranes of myoblasts in developing skeletal muscles of mice.Acta Histochem. 2020 Apr;122(3):151513. doi: 10.1016/j.acthis.2020.151513. Epub 2020 Feb 12. Acta Histochem. 2020. PMID: 32059926
-
Differential myogenicity of satellite cells isolated from extensor digitorum longus (EDL) and soleus rat muscles revealed in vitro.Cell Tissue Res. 1998 Mar;291(3):455-68. doi: 10.1007/s004410051015. Cell Tissue Res. 1998. PMID: 9477302
-
Isolation, Culture, and Immunostaining of Skeletal Muscle Myofibers from Wildtype and Nestin-GFP Mice as a Means to Analyze Satellite Cell.Methods Mol Biol. 2017;1556:51-102. doi: 10.1007/978-1-4939-6771-1_4. Methods Mol Biol. 2017. PMID: 28247345
Cited by
-
Terminal Schwann Cell Aging: Implications for Age-Associated Neuromuscular Dysfunction.Aging Dis. 2021 Apr 1;12(2):494-514. doi: 10.14336/AD.2020.0708. eCollection 2021 Apr. Aging Dis. 2021. PMID: 33815879 Free PMC article. Review.
-
Major Reorganization of Chromosome Conformation During Muscle Development in Pig.Front Genet. 2021 Oct 5;12:748239. doi: 10.3389/fgene.2021.748239. eCollection 2021. Front Genet. 2021. PMID: 34675966 Free PMC article.
-
Calmodulin-Binding Proteins in Muscle: A Minireview on Nuclear Receptor Interacting Protein, Neurogranin, and Growth-Associated Protein 43.Int J Mol Sci. 2020 Feb 4;21(3):1016. doi: 10.3390/ijms21031016. Int J Mol Sci. 2020. PMID: 32033037 Free PMC article. Review.
-
New insights into the relationship between mIGF-1-induced hypertrophy and Ca2+ handling in differentiated satellite cells.PLoS One. 2014 Sep 17;9(9):e107753. doi: 10.1371/journal.pone.0107753. eCollection 2014. PLoS One. 2014. PMID: 25229238 Free PMC article.
-
Specific association of growth-associated protein 43 with calcium release units in skeletal muscles of lower vertebrates.Eur J Histochem. 2014 Dec 5;58(4):2453. doi: 10.4081/ejh.2014.2453. Eur J Histochem. 2014. PMID: 25578978 Free PMC article.
References
-
- Oestreicher AB, Zwiers H, Gispen WH, Roberts S (1982) Characterization of infant rat cerebral cortical membrane proteins phosphorylated in vivo: identification of the ACTH-sensitive phosphoprotein B-50. J Neurochem 39: 683–692. - PubMed
-
- Oestreicher AB, Zwiers H, Schotman P, Gispen WH (1981) Immunohistochemical localization of a phosphoprotein (B-50) isolated from rat brain synaptosomal plasma membranes. Brain Res Bull 6: 145–153. - PubMed
-
- Mosevitsky MI (2005) Nerve ending “signal” proteins GAP-43, MARCKS, and BASP1. Int Rev Cytol 245: 245–325. - PubMed
-
- Dent EW, Meiri KF (1998) Distribution of phosphorylated GAP-43 (neuromodulin) in growth cones directly reflects growth cone behavior. J Neurobiol 35: 287–299. - PubMed
-
- Korshunova I, Novitskaya V, Kiryushko D, Pedersen N, Kolkova K, et al. (2007) GAP-43 regulates NCAM-180-mediated neurite outgrowth. J Neurochem 100: 1599–1612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

