Dual function of novel pollen coat (surface) proteins: IgE-binding capacity and proteolytic activity disrupting the airway epithelial barrier
- PMID: 23308195
- PMCID: PMC3538775
- DOI: 10.1371/journal.pone.0053337
Dual function of novel pollen coat (surface) proteins: IgE-binding capacity and proteolytic activity disrupting the airway epithelial barrier
Abstract
Background: The pollen coat is the first structure of the pollen to encounter the mucosal immune system upon inhalation. Prior characterizations of pollen allergens have focused on water-soluble, cytoplasmic proteins, but have overlooked much of the extracellular pollen coat. Due to washing with organic solvents when prepared, these pollen coat proteins are typically absent from commercial standardized allergenic extracts (i.e., "de-fatted"), and, as a result, their involvement in allergy has not been explored.
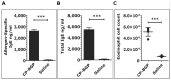
Methodology/principal findings: Using a unique approach to search for pollen allergenic proteins residing in the pollen coat, we employed transmission electron microscopy (TEM) to assess the impact of organic solvents on the structural integrity of the pollen coat. TEM results indicated that de-fatting of Cynodon dactylon (Bermuda grass) pollen (BGP) by use of organic solvents altered the structural integrity of the pollen coat. The novel IgE-binding proteins of the BGP coat include a cysteine protease (CP) and endoxylanase (EXY). The full-length cDNA that encodes the novel IgE-reactive CP was cloned from floral RNA. The EXY and CP were purified to homogeneity and tested for IgE reactivity. The CP from the BGP coat increased the permeability of human airway epithelial cells, caused a clear concentration-dependent detachment of cells, and damaged their barrier integrity.
Conclusions/significance: Using an immunoproteomics approach, novel allergenic proteins of the BGP coat were identified. These proteins represent a class of novel dual-function proteins residing on the coat of the pollen grain that have IgE-binding capacity and proteolytic activity, which disrupts the integrity of the airway epithelial barrier. The identification of pollen coat allergens might explain the IgE-negative response to available skin-prick-testing proteins in patients who have positive symptoms. Further study of the role of these pollen coat proteins in allergic responses is warranted and could potentially lead to the development of improved diagnostic and therapeutic tools.
Conflict of interest statement
Figures




References
-
- Stingone JA, Claudio L (2008) Disparities in allergy testing and health outcomes among urban children with asthma. J Allergy Clin Immunol 122: 748–753. - PubMed
-
- Suvilehto J, Seppanen M, Notkola IL, Antikainen M, Malmberg H, et al. (2007) Association of allergy, asthma and IgE sensitisation to adenoidectomy and infections in children. Rhinology 45: 286–291. - PubMed
-
- Nathan RA (2007) The burden of allergic rhinitis. Allergy Asthma Proc 28: 3–9. - PubMed
-
- Verdino P (2006) Structural characterization of pollen allergens. Clinical Reviews in Allergy & Immunology 30: 73–95. - PubMed
-
- Tao AL, He SH (2005) Cloning, expression, and characterization of pollen allergens from Humulus scandens (Lour) Merr and Ambrosia artemisiifolia L. Acta Pharmacol Sin. 26: 1225–1232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

