Self-interaction of human Pex11pβ during peroxisomal growth and division
- PMID: 23308220
- PMCID: PMC3538539
- DOI: 10.1371/journal.pone.0053424
Self-interaction of human Pex11pβ during peroxisomal growth and division
Abstract
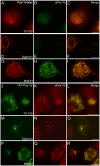
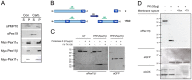
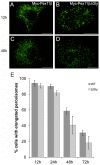
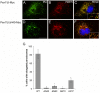
Pex11 proteins are involved in membrane elongation and division processes associated with the multiplication of peroxisomes. Human Pex11pβ has recently been linked to a new disorder affecting peroxisome morphology and dynamics. Here, we have analyzed the exact membrane topology of Pex11pβ. Studies with an epitope-specific antibody and protease protection assays show that Pex11pβ is an integral membrane protein with two transmembrane domains flanking an internal region exposed to the peroxisomal matrix and N- and C-termini facing the cytosol. A glycine-rich internal region within Pex11pβ is dispensable for peroxisome membrane elongation and division. However, we demonstrate that an amphipathic helix (Helix 2) within the first N-terminal 40 amino acids is crucial for membrane elongation and self-interaction of Pex11pβ. Interestingly, we find that Pex11pβ self-interaction strongly depends on the detergent used for solubilization. We also show that N-terminal cysteines are not essential for membrane elongation, and that putative N-terminal phosphorylation sites are dispensable for Pex11pβ function. We propose that self-interaction of Pex11pβ regulates its membrane deforming activity in conjunction with membrane lipids.
Conflict of interest statement
Figures



Similar articles
-
Pex11pbeta-mediated growth and division of mammalian peroxisomes follows a maturation pathway.J Cell Sci. 2010 Aug 15;123(Pt 16):2750-62. doi: 10.1242/jcs.062109. Epub 2010 Jul 20. J Cell Sci. 2010. PMID: 20647371
-
Membrane curvature during peroxisome fission requires Pex11.EMBO J. 2011 Jan 5;30(1):5-16. doi: 10.1038/emboj.2010.299. Epub 2010 Nov 26. EMBO J. 2011. PMID: 21113128 Free PMC article.
-
Fis1, DLP1, and Pex11p coordinately regulate peroxisome morphogenesis.Exp Cell Res. 2007 May 1;313(8):1675-86. doi: 10.1016/j.yexcr.2007.02.028. Epub 2007 Mar 12. Exp Cell Res. 2007. PMID: 17408615
-
Peroxisomes: membrane events accompanying peroxisome proliferation.Int J Biochem Cell Biol. 2011 Jun;43(6):847-51. doi: 10.1016/j.biocel.2011.03.006. Epub 2011 Mar 17. Int J Biochem Cell Biol. 2011. PMID: 21419861 Review.
-
Biogenesis of peroxisomes. Topogenesis of the peroxisomal membrane and matrix proteins.FEBS J. 2005 May;272(10):2362-72. doi: 10.1111/j.1742-4658.2005.04690.x. FEBS J. 2005. PMID: 15885087 Review.
Cited by
-
PPARgamma dependent PEX11beta counteracts the suppressive role of SIRT1 on neural differentiation of HESCs.PLoS One. 2024 May 16;19(5):e0298274. doi: 10.1371/journal.pone.0298274. eCollection 2024. PLoS One. 2024. PMID: 38753762 Free PMC article.
-
Post-translational modifications of proteins associated with yeast peroxisome membrane: An essential mode of regulatory mechanism.Genes Cells. 2021 Nov;26(11):843-860. doi: 10.1111/gtc.12892. Epub 2021 Sep 2. Genes Cells. 2021. PMID: 34472666 Free PMC article. Review.
-
Giant peroxisomes in a moss (Physcomitrella patens) peroxisomal biogenesis factor 11 mutant.New Phytol. 2016 Jan;209(2):576-89. doi: 10.1111/nph.13739. Epub 2015 Nov 6. New Phytol. 2016. PMID: 26542980 Free PMC article.
-
The membrane remodeling protein Pex11p activates the GTPase Dnm1p during peroxisomal fission.Proc Natl Acad Sci U S A. 2015 May 19;112(20):6377-82. doi: 10.1073/pnas.1418736112. Epub 2015 May 4. Proc Natl Acad Sci U S A. 2015. PMID: 25941407 Free PMC article.
-
Fission Impossible (?)-New Insights into Disorders of Peroxisome Dynamics.Cells. 2022 Jun 14;11(12):1922. doi: 10.3390/cells11121922. Cells. 2022. PMID: 35741050 Free PMC article. Review.
References
-
- Wanders RJ, Waterham HR (2006) Peroxisomal disorders: the single peroxisomal enzyme deficiencies. Biochim Biophys Acta 1763: 1707–1720. - PubMed
-
- Islinger M, Grille S, Fahimi HD, Schrader M (2012) The peroxisome: an update on mysteries. Histochem Cell Biol 137: 547–574. - PubMed
-
- Lizard G, Rouaud O, Demarquoy J, Cherkaoui-Malki M, Iuliano L (2012) Potential Roles of Peroxisomes in Alzheimer's Disease and in Dementia of the Alzheimer's Type. J Alzheimers Dis 29: 241–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

