Conditional deletion of the Pten gene in the mouse prostate induces prostatic intraepithelial neoplasms at early ages but a slow progression to prostate tumors
- PMID: 23308230
- PMCID: PMC3540073
- DOI: 10.1371/journal.pone.0053476
Conditional deletion of the Pten gene in the mouse prostate induces prostatic intraepithelial neoplasms at early ages but a slow progression to prostate tumors
Abstract
The PTEN tumor suppressor gene is frequently inactivated in human prostate cancer. Using Osr1 (odd skipped related 1)-Cre mice, we generated a novel conditional Pten knockout mouse strain, Pten(LoxP):Osr1-Cre. Conditional biallelic and monoallelic Pten knockout mice were viable. Deletion of Pten expression was detected in the prostate of Pten(LoxP/LoxP):Osr1-Cre mice as early as 2 weeks of age. Intriguingly, Pten(LoxP/LoxP):Osr1-Cre mice develop high-grade prostatic intraepithelial neoplasms (PINs) with high penetrance as early as one-month of age, and locally invasive prostatic tumors after 12-months of age. Pten(LoxP/+):Osr1-Cre mice show only mild oncogenic changes after 8-weeks of age. Castration of Pten(LoxP/LoxP):Osr1-Cre mice shows no significant regression of prostate tumors, although a shift of androgen receptor (AR) staining from the nuclei to cytoplasm is observed in Pten null tumor cells of castrated mice. Enhanced Akt activity is observed in Pten null tumor cells of castrated Pten(LoxP/LoxP):Osr1-Cre. This study provides a novel mouse model that can be used to investigate a primary role of Pten in initiating oncogenic transformation in the prostate and to examine other genetic and epigenetic changes that are required for tumor progression in the mouse prostate.
Conflict of interest statement
Figures
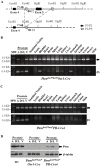
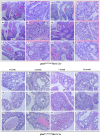
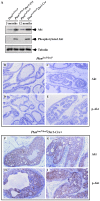
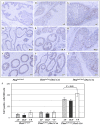



Similar articles
-
Conditional expression of the androgen receptor induces oncogenic transformation of the mouse prostate.J Biol Chem. 2011 Sep 23;286(38):33478-88. doi: 10.1074/jbc.M111.269894. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795710 Free PMC article.
-
The pace of prostatic intraepithelial neoplasia development is determined by the timing of Pten tumor suppressor gene excision.PLoS One. 2008;3(12):e3940. doi: 10.1371/journal.pone.0003940. Epub 2008 Dec 15. PLoS One. 2008. PMID: 19081794 Free PMC article.
-
Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis.Cancer Res. 2005 Jul 1;65(13):5730-9. doi: 10.1158/0008-5472.CAN-04-4519. Cancer Res. 2005. PMID: 15994948
-
Advances in mouse models of prostate cancer.Expert Rev Mol Med. 2008 Jun 9;10:e16. doi: 10.1017/S1462399408000689. Expert Rev Mol Med. 2008. PMID: 18538039 Review.
-
Epidemiology and molecular biology of early prostatic neoplasia.Mol Urol. 2000 Fall;4(3):109-13;discussion 115. Mol Urol. 2000. PMID: 11062364 Review.
Cited by
-
The comprehensive role of E-cadherin in maintaining prostatic epithelial integrity during oncogenic transformation and tumor progression.PLoS Genet. 2019 Oct 28;15(10):e1008451. doi: 10.1371/journal.pgen.1008451. eCollection 2019 Oct. PLoS Genet. 2019. PMID: 31658259 Free PMC article.
-
A clinical study on the expression of PTEN in renal cell carcinoma in children.Oncol Lett. 2019 Jan;17(1):69-72. doi: 10.3892/ol.2018.9571. Epub 2018 Oct 12. Oncol Lett. 2019. PMID: 30655739 Free PMC article.
-
Activation of hepatocyte growth factor/MET signaling initiates oncogenic transformation and enhances tumor aggressiveness in the murine prostate.J Biol Chem. 2018 Dec 28;293(52):20123-20136. doi: 10.1074/jbc.RA118.005395. Epub 2018 Nov 6. J Biol Chem. 2018. PMID: 30401749 Free PMC article.
-
FDPS cooperates with PTEN loss to promote prostate cancer progression through modulation of small GTPases/AKT axis.Oncogene. 2019 Jun;38(26):5265-5280. doi: 10.1038/s41388-019-0791-9. Epub 2019 Mar 26. Oncogene. 2019. PMID: 30914801 Free PMC article.
-
Melittin restores PTEN expression by down-regulating HDAC2 in human hepatocelluar carcinoma HepG2 cells.PLoS One. 2014 May 2;9(5):e95520. doi: 10.1371/journal.pone.0095520. eCollection 2014. PLoS One. 2014. PMID: 24788349 Free PMC article.
References
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, et al. (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275: 1943–1947. - PubMed
-
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, et al. (1997) Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15: 356–362. - PubMed
-
- Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

