Evidence that the EphA2 receptor exacerbates ischemic brain injury
- PMID: 23308246
- PMCID: PMC3538581
- DOI: 10.1371/journal.pone.0053528
Evidence that the EphA2 receptor exacerbates ischemic brain injury
Abstract
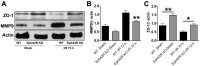
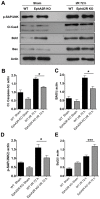
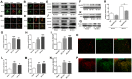
Ephrin (Eph) signaling within the central nervous system is known to modulate axon guidance, synaptic plasticity, and to promote long-term potentiation. We investigated the potential involvement of EphA2 receptors in ischemic stroke-induced brain inflammation in a mouse model of focal stroke. Cerebral ischemia was induced in male C57Bl6/J wild-type (WT) and EphA2-deficient (EphA2(-/-)) mice by middle cerebral artery occlusion (MCAO; 60 min), followed by reperfusion (24 or 72 h). Brain infarction was measured using triphenyltetrazolium chloride staining. Neurological deficit scores and brain infarct volumes were significantly less in EphA2(-/-) mice compared with WT controls. This protection by EphA2 deletion was associated with a comparative decrease in brain edema, blood-brain barrier damage, MMP-9 expression and leukocyte infiltration, and higher expression levels of the tight junction protein, zona occludens-1. Moreover, EphA2(-/-) brains had significantly lower levels of the pro-apoptotic proteins, cleaved caspase-3 and BAX, and higher levels of the anti-apoptotic protein, Bcl-2 as compared to WT group. We confirmed that isolated WT cortical neurons express the EphA2 receptor and its ligands (ephrin-A1-A3). Furthermore, expression of all four proteins was increased in WT primary cortical neurons following 24 h of glucose deprivation, and in the brains of WT mice following stroke. Glucose deprivation induced less cell death in primary neurons from EphA2(-/-) compared with WT mice. In conclusion, our data provide the first evidence that the EphA2 receptor directly contributes to blood-brain barrier damage and neuronal death following ischemic stroke.
Conflict of interest statement
Figures

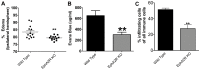
Similar articles
-
Neuropilin 2 deficiency does not affect cortical neuronal viability in response to oxygen-glucose-deprivation and transient middle cerebral artery occlusion.Neurosci Res. 2010 Apr;66(4):396-401. doi: 10.1016/j.neures.2009.12.010. Epub 2009 Dec 28. Neurosci Res. 2010. PMID: 20036291
-
Effect of PPAR-β/δ agonist GW0742 treatment in the acute phase response and blood-brain barrier permeability following brain injury.Transl Res. 2017 Apr;182:27-48. doi: 10.1016/j.trsl.2016.10.004. Epub 2016 Oct 18. Transl Res. 2017. PMID: 27818230
-
Na(+)-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia.J Cereb Blood Flow Metab. 2005 Jan;25(1):54-66. doi: 10.1038/sj.jcbfm.9600006. J Cereb Blood Flow Metab. 2005. PMID: 15678112
-
α(2)-adrenoceptors do not mediate neuroprotection in acute ischemic stroke in mice.J Cereb Blood Flow Metab. 2011 Oct;31(10):e1-7. doi: 10.1038/jcbfm.2011.110. Epub 2011 Jul 27. J Cereb Blood Flow Metab. 2011. PMID: 21792243 Free PMC article. Review.
-
Toll-like receptor signaling in endogenous neuroprotection and stroke.Neuroscience. 2009 Feb 6;158(3):1007-20. doi: 10.1016/j.neuroscience.2008.07.067. Epub 2008 Aug 12. Neuroscience. 2009. PMID: 18809468 Free PMC article. Review.
Cited by
-
The Blood-Brain Barrier and the EphR/Ephrin System: Perspectives on a Link Between Neurovascular and Neuropsychiatric Disorders.Front Mol Neurosci. 2018 Apr 12;11:127. doi: 10.3389/fnmol.2018.00127. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29706868 Free PMC article. Review.
-
PKA Inhibitor H89 (N-[2-p-bromocinnamylamino-ethyl]-5-isoquinolinesulfonamide) Attenuates Synaptic Dysfunction and Neuronal Cell Death following Ischemic Injury.Neural Plast. 2015;2015:374520. doi: 10.1155/2015/374520. Epub 2015 Sep 13. Neural Plast. 2015. PMID: 26448879 Free PMC article.
-
The Role of Progranulin (PGRN) in the Pathogenesis of Ischemic Stroke.Cell Mol Neurobiol. 2023 Oct;43(7):3435-3447. doi: 10.1007/s10571-023-01396-8. Epub 2023 Aug 10. Cell Mol Neurobiol. 2023. PMID: 37561339 Free PMC article. Review.
-
Approaches to Manipulate Ephrin-A:EphA Forward Signaling Pathway.Pharmaceuticals (Basel). 2020 Jun 30;13(7):140. doi: 10.3390/ph13070140. Pharmaceuticals (Basel). 2020. PMID: 32629797 Free PMC article. Review.
-
Vascular Sema3E-Plexin-D1 Signaling Reactivation Promotes Post-stroke Recovery through VEGF Downregulation in Mice.Transl Stroke Res. 2022 Feb;13(1):142-159. doi: 10.1007/s12975-021-00914-4. Epub 2021 May 12. Transl Stroke Res. 2022. PMID: 33978913 Free PMC article.
References
-
- Davy A, Soriano P (2005) Ephrin signaling in vivo: look both ways. Dev Dyn 232: 1–10. - PubMed
-
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, et al. (1996) Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 17: 9–19. - PubMed
-
- Himanen JP, Nikolov DB (2003) Eph signaling: a structural view. Trends Neurosci 26: 46–51. - PubMed
-
- Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133: 38–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

