A specialized microvascular domain in the mouse neural stem cell niche
- PMID: 23308251
- PMCID: PMC3538546
- DOI: 10.1371/journal.pone.0053546
A specialized microvascular domain in the mouse neural stem cell niche
Abstract
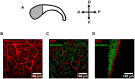
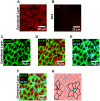
The microenvironment of the subependymal zone (SEZ) neural stem cell niche is necessary for regulating adult neurogenesis. In particular, signaling from the microvasculature is essential for adult neural stem cell maintenance, but microvascular structure and blood flow dynamics in the SEZ are not well understood. In this work, we show that the mouse SEZ constitutes a specialized microvascular domain defined by unique vessel architecture and reduced rates of blood flow. Additionally, we demonstrate that hypoxic conditions are detectable in the ependymal layer that lines the ventricle, and in a subpopulation of neurons throughout the SEZ and striatum. Together, these data highlight previously unidentified features of the SEZ neural stem cell niche, and further demonstrate the extent of microvascular specialization in the SEZ microenvironment.
Conflict of interest statement
Figures






Similar articles
-
LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche.J Cell Sci. 2010 Jun 1;123(Pt 11):1922-30. doi: 10.1242/jcs.065912. Epub 2010 May 11. J Cell Sci. 2010. PMID: 20460439
-
The number of stem cells in the subependymal zone of the adult rodent brain is correlated with the number of ependymal cells and not with the volume of the niche.Stem Cells Dev. 2012 May 1;21(7):1090-6. doi: 10.1089/scd.2011.0130. Epub 2011 Sep 7. Stem Cells Dev. 2012. PMID: 21762017 Free PMC article.
-
Lesion-induced accumulation of platelets promotes survival of adult neural stem / progenitor cells.Exp Neurol. 2015 Jul;269:75-89. doi: 10.1016/j.expneurol.2015.03.018. Epub 2015 Mar 24. Exp Neurol. 2015. PMID: 25819103
-
Paracrine regulation of neural stem cells in the subependymal zone.Arch Biochem Biophys. 2013 Jun;534(1-2):11-9. doi: 10.1016/j.abb.2012.10.001. Epub 2012 Oct 13. Arch Biochem Biophys. 2013. PMID: 23073070 Review.
-
Physiological Interactions between Microglia and Neural Stem Cells in the Adult Subependymal Niche.Neuroscience. 2019 May 1;405:77-91. doi: 10.1016/j.neuroscience.2019.01.009. Epub 2019 Jan 21. Neuroscience. 2019. PMID: 30677487 Review.
Cited by
-
Isolation of neural stem and oligodendrocyte progenitor cells from the brain of live rats.Stem Cell Reports. 2021 Oct 12;16(10):2534-2547. doi: 10.1016/j.stemcr.2021.08.015. Epub 2021 Sep 23. Stem Cell Reports. 2021. PMID: 34560001 Free PMC article.
-
Regulation of tissue morphogenesis by endothelial cell-derived signals.Trends Cell Biol. 2015 Mar;25(3):148-57. doi: 10.1016/j.tcb.2014.11.007. Epub 2014 Dec 17. Trends Cell Biol. 2015. PMID: 25529933 Free PMC article. Review.
-
Current Understanding of the Neural Stem Cell Niches.Cells. 2022 Sep 26;11(19):3002. doi: 10.3390/cells11193002. Cells. 2022. PMID: 36230964 Free PMC article. Review.
-
Rapid transport of CCL11 across the blood-brain barrier: regional variation and importance of blood cells.J Pharmacol Exp Ther. 2014 Jun;349(3):497-507. doi: 10.1124/jpet.114.213074. Epub 2014 Apr 4. J Pharmacol Exp Ther. 2014. PMID: 24706984 Free PMC article.
-
Neural stem cell niche heterogeneity.Semin Cell Dev Biol. 2019 Nov;95:42-53. doi: 10.1016/j.semcdb.2019.01.005. Epub 2019 Jan 14. Semin Cell Dev Biol. 2019. PMID: 30639325 Free PMC article. Review.
References
-
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, et al. (1998) Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317. - PubMed
-
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716. - PubMed
-
- Palmer TD, Willhoite AR, Gage FH (2000) Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425: 479–494. - PubMed
-
- Rakic P (2003) Elusive radial glial cells: historical and evolutionary perspective. Glia 43: 19–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

