Chlamydia induces anchorage independence in 3T3 cells and detrimental cytological defects in an infection model
- PMID: 23308295
- PMCID: PMC3538680
- DOI: 10.1371/journal.pone.0054022
Chlamydia induces anchorage independence in 3T3 cells and detrimental cytological defects in an infection model
Abstract
Chlamydia are gram negative, obligate intracellular bacterial organisms with different species causing a multitude of infections in both humans and animals. Chlamydia trachomatis is the causative agent of the sexually transmitted infection (STI) Chlamydia, the most commonly acquired bacterial STI in the United States. Chlamydial infections have also been epidemiologically linked to cervical cancer in women co-infected with the human papillomavirus (HPV). We have previously shown chlamydial infection results in centrosome amplification and multipolar spindle formation leading to chromosomal instability. Many studies indicate that centrosome abnormalities, spindle defects, and chromosome segregation errors can lead to cell transformation. We hypothesize that the presence of these defects within infected dividing cells identifies a possible mechanism for Chlamydia as a cofactor in cervical cancer formation. Here we demonstrate that infection with Chlamydia trachomatis is able to transform 3T3 cells in soft agar resulting in anchorage independence and increased colony formation. Additionally, we show for the first time Chlamydia infects actively replicating cells in vivo. Infection of mice with Chlamydia results in significantly increased cell proliferation within the cervix, and in evidence of cervical dysplasia. Confocal examination of these infected tissues also revealed elements of chlamydial induced chromosome instability. These results contribute to a growing body of data implicating a role for Chlamydia in cervical cancer development and suggest a possible molecular mechanism for this effect.
Conflict of interest statement
Figures
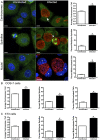

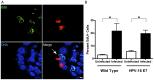


Similar articles
-
Chlamydia trachomatis infection causes mitotic spindle pole defects independently from its effects on centrosome amplification.Traffic. 2011 Jul;12(7):854-66. doi: 10.1111/j.1600-0854.2011.01204.x. Epub 2011 May 12. Traffic. 2011. PMID: 21477082 Free PMC article.
-
Chlamydia trachomatis modulates expression of tumor suppressor gene caveolin-1 and oncogene C-myc in the transformation zone of non-neoplastic cervical tissue.Gynecol Oncol. 2005 Sep;98(3):409-19. doi: 10.1016/j.ygyno.2005.04.034. Gynecol Oncol. 2005. PMID: 16005053
-
Chlamydia trachomatis and human papillomavirus infection in Indian women with sexually transmitted diseases and cervical precancerous and cancerous lesions.Clin Microbiol Infect. 2000 Feb;6(2):88-93. doi: 10.1046/j.1469-0691.2000.00024.x. Clin Microbiol Infect. 2000. PMID: 11168078
-
[The role of chlamydia trachomatis infection in CIN and cervical cancer development].Ginekol Pol. 2002 May;73(5):472-6. Ginekol Pol. 2002. PMID: 12185710 Review. Polish.
-
[Association of Chlamydia trachomatis and human papilloma virus as predisposing factors in cervical intraepithelial neoplasia].Ginecol Obstet Mex. 1995 Oct;63:422-6. Ginecol Obstet Mex. 1995. PMID: 8549925 Review. Spanish.
Cited by
-
Subversion of host genome integrity by bacterial pathogens.Nat Rev Mol Cell Biol. 2016 Oct;17(10):659-73. doi: 10.1038/nrm.2016.100. Epub 2016 Aug 18. Nat Rev Mol Cell Biol. 2016. PMID: 27534801 Review.
-
Sulforaphane promotes chlamydial infection by suppressing mitochondrial protein oxidation and activation of complement C3.Protein Sci. 2019 Jan;28(1):216-227. doi: 10.1002/pro.3536. Protein Sci. 2019. PMID: 30367535 Free PMC article.
-
Deregulation of the cyclin-dependent kinase inhibitor p27 as a putative candidate for transformation in Chlamydia trachomatis infected mesenchymal stem cells.AIMS Microbiol. 2023 Feb 28;9(1):131-150. doi: 10.3934/microbiol.2023009. eCollection 2023. AIMS Microbiol. 2023. PMID: 36891539 Free PMC article.
-
Chlamydia and HPV induce centrosome amplification in the host cell through additive mechanisms.Cell Microbiol. 2021 Dec;23(12):e13397. doi: 10.1111/cmi.13397. Epub 2021 Nov 10. Cell Microbiol. 2021. PMID: 34716742 Free PMC article.
-
Chlamydia trachomatis Genital Infections.Microb Cell. 2016 Sep 5;3(9):390-403. doi: 10.15698/mic2016.09.525. Microb Cell. 2016. PMID: 28357377 Free PMC article. Review.
References
-
- Bébéar C, de Barbeyrac B (2009) Genital Chlamydia trachomatis infections. Clin Microbiol Infect 15: 4–10 doi:10.1111/j.1469-0691.2008.02647.x. - DOI - PubMed
-
- Ohman H, Tiitinen A, Halttunen M, Lehtinen M, Paavonen J, et al. (2009) Cytokine polymorphisms and severity of tubal damage in women with Chlamydia-associated infertility. J Infect Dis 199: 1353–1359 doi:10.1086/597620. - DOI - PubMed
-
- Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, et al. (2004) Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291: 2229–2236 doi:10.1001/jama.291.18.2229. - DOI - PubMed
-
- Datta SD, Torrone E, Kruszon-Moran D, Berman S, Johnson R, et al. (2012) Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999–2008. Sex Transm Dis 39: 92–96 doi:10.1097/OLQ.0b013e31823e2ff7. - DOI - PubMed
-
- World Health Organization Department of HIV/AIDS © 2001 (2001) Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates: 1–50.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

