Screening for drug-induced hepatotoxicity in primary mouse hepatocytes using acetaminophen, amiodarone, and cyclosporin a as model compounds: an omics-guided approach
- PMID: 23308384
- PMCID: PMC3567623
- DOI: 10.1089/omi.2012.0079
Screening for drug-induced hepatotoxicity in primary mouse hepatocytes using acetaminophen, amiodarone, and cyclosporin a as model compounds: an omics-guided approach
Abstract
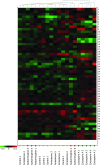
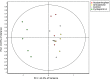
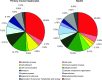
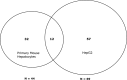
Drug-induced hepatotoxicity is a leading cause of attrition for candidate pharmaceuticals in development. New preclinical screening methods are crucial to predict drug toxicity prior to human studies. Of all in vitro hepatotoxicity models, primary human hepatocytes are considered as 'the gold standard.' However, their use is hindered by limited availability and inter-individual variation. These barriers may be overcome by using primary mouse hepatocytes. We used differential in gel electrophoresis (DIGE) to study large-scale protein expression of primary mouse hepatocytes. These hepatocytes were exposed to three well-defined hepatotoxicants: acetaminophen, amiodarone, and cyclosporin A. Each hepatotoxicant induces a different hepatotoxic phenotype. Based on the DIGE results, the mRNA expression levels of deregulated proteins from cyclosporin A-treated cells were also analyzed. We were able to distinguish cyclosporin A from controls, as well as acetaminophen and amiodarone-treated samples. Cyclosporin A induced endoplasmic reticulum (ER) stress and altered the ER-Golgi transport. Moreover, liver carboxylesterase and bile salt sulfotransferase were differentially expressed. These proteins were associated with a protective adaptive response against cyclosporin A-induced cholestasis. The results of this study are comparable with effects in HepG2 cells. Therefore, we suggest both models can be used to analyze the cholestatic properties of cyclosporin A. Furthermore, this study showed a conserved response between primary mouse hepatocytes and HepG2 cells. These findings collectively lend support for use of omics strategies in preclinical toxicology, and might inform future efforts to better link preclinical and clinical research in rational drug development.
Figures


Similar articles
-
Integrative cross-omics analysis in primary mouse hepatocytes unravels mechanisms of cyclosporin A-induced hepatotoxicity.Toxicology. 2014 Oct 3;324:18-26. doi: 10.1016/j.tox.2014.06.003. Epub 2014 Jul 15. Toxicology. 2014. PMID: 25047351
-
Proteomics investigations of drug-induced hepatotoxicity in HepG2 cells.Toxicol Sci. 2011 Mar;120(1):109-22. doi: 10.1093/toxsci/kfq380. Epub 2010 Dec 16. Toxicol Sci. 2011. PMID: 21163907
-
A transcriptomics-based hepatotoxicity comparison between the zebrafish embryo and established human and rodent in vitro and in vivo models using cyclosporine A, amiodarone and acetaminophen.Toxicol Lett. 2015 Jan 22;232(2):403-12. doi: 10.1016/j.toxlet.2014.11.020. Epub 2014 Nov 24. Toxicol Lett. 2015. PMID: 25448281
-
Validation of gene expression profiles from cholestatic hepatotoxicants in vitro against human in vivo cholestasis.Toxicol In Vitro. 2017 Oct;44:322-329. doi: 10.1016/j.tiv.2017.07.024. Epub 2017 Aug 1. Toxicol In Vitro. 2017. PMID: 28778767
-
Pathophysiological relevance of proteomics investigations of drug-induced hepatotoxicity in HepG2 cells.Toxicol Sci. 2011 Jun;121(2):428-30; author reply 431-3. doi: 10.1093/toxsci/kfr053. Epub 2011 Mar 7. Toxicol Sci. 2011. PMID: 21385733 Free PMC article. No abstract available.
Cited by
-
The human hepatocyte TXG-MAPr: gene co-expression network modules to support mechanism-based risk assessment.Arch Toxicol. 2021 Dec;95(12):3745-3775. doi: 10.1007/s00204-021-03141-w. Epub 2021 Oct 9. Arch Toxicol. 2021. PMID: 34626214 Free PMC article.
-
Age-related cataracts: Role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection.Prog Retin Eye Res. 2017 Sep;60:1-19. doi: 10.1016/j.preteyeres.2017.08.003. Epub 2017 Aug 31. Prog Retin Eye Res. 2017. PMID: 28864287 Free PMC article. Review.
-
Increased liver-specific proteins in circulating extracellular vesicles as potential biomarkers for drug- and alcohol-induced liver injury.PLoS One. 2017 Feb 22;12(2):e0172463. doi: 10.1371/journal.pone.0172463. eCollection 2017. PLoS One. 2017. PMID: 28225807 Free PMC article.
-
Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues.Mol Cell Proteomics. 2015 May;14(5):1400-10. doi: 10.1074/mcp.M114.044305. Epub 2015 Feb 27. Mol Cell Proteomics. 2015. PMID: 25724911 Free PMC article.
-
Drug-induced steatohepatitis.Expert Opin Drug Metab Toxicol. 2017 Feb;13(2):193-204. doi: 10.1080/17425255.2017.1246534. Epub 2016 Oct 27. Expert Opin Drug Metab Toxicol. 2017. PMID: 27759439 Free PMC article. Review.
References
-
- Aardema MJ. MacGregor JT. Toxicology and genetic toxicology in the new era of “toxicogenomics”: Impact of “-omics” technologies. Mutat Res. 2002;499:13–25. - PubMed
-
- Aaseth J. Thomassen Y. Aadland E. Fausa O. Schrumpf E. Hepatic retention of copper and selenium in primary sclerosing cholangitis. Scand J Gastroenterol. 1995;30:1200–1203. - PubMed
-
- Alrefai WA. Gill RK. Bile acid transporters: Structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24:1803–1823. - PubMed
-
- Belin MW. Bouchard CS. Phillips TM. Update on topical cyclosporin A. Background, immunology, and pharmacology. Cornea. 1990;9:184–195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

