A kinetic model for RNA-interference of focal adhesions
- PMID: 23311633
- PMCID: PMC3616989
- DOI: 10.1186/1752-0509-7-2
A kinetic model for RNA-interference of focal adhesions
Abstract
Background: Focal adhesions are integrin-based cell-matrix contacts that transduce and integrate mechanical and biochemical cues from the environment. They develop from smaller and more numerous focal complexes under the influence of mechanical force and are key elements for many physiological and disease-related processes, including wound healing and metastasis. More than 150 different proteins localize to focal adhesions and have been systematically classified in the adhesome project (http://www.adhesome.org). First RNAi-screens have been performed for focal adhesions and the effect of knockdown of many of these components on the number, size, shape and location of focal adhesions has been reported.
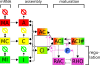
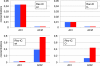
Results: We have developed a kinetic model for RNA interference of focal adhesions which represents some of its main elements: a spatially layered structure, signaling through the small GTPases Rac and Rho, and maturation from focal complexes to focal adhesions under force. The response to force is described by two complementary scenarios corresponding to slip and catch bond behavior, respectively. Using estimated and literature values for the model parameters, three time scales of the dynamics of RNAi-influenced focal adhesions are identified: a sub-minute time scale for the assembly of focal complexes, a sub-hour time scale for the maturation to focal adhesions, and a time scale of days that controls the siRNA-mediated knockdown. Our model shows bistability between states dominated by focal complexes and focal adhesions, respectively. Catch bonding strongly extends the range of stability of the state dominated by focal adhesions. A sensitivity analysis predicts that knockdown of focal adhesion components is more efficient for focal adhesions with slip bonds or if the system is in a state dominated by focal complexes. Knockdown of Rho leads to an increase of focal complexes.
Conclusions: The suggested model provides a kinetic description of the effect of RNA-interference of focal adhesions. Its predictions are in good agreement with known experimental results and can now guide the design of RNAi-experiments. In the future, it can be extended to include more components of the adhesome. It also could be extended by spatial aspects, for example by the differential activation of the Rac- and Rho-pathways in different parts of the cell.
Figures







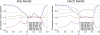
Similar articles
-
Paxillin phosphorylation at serine 273 and its effects on Rac, Rho and adhesion dynamics.PLoS Comput Biol. 2018 Jul 5;14(7):e1006303. doi: 10.1371/journal.pcbi.1006303. eCollection 2018 Jul. PLoS Comput Biol. 2018. PMID: 29975690 Free PMC article.
-
Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism.J Cell Biol. 2001 Jun 11;153(6):1175-86. doi: 10.1083/jcb.153.6.1175. J Cell Biol. 2001. PMID: 11402062 Free PMC article.
-
Model of coupled transient changes of Rac, Rho, adhesions and stress fibers alignment in endothelial cells responding to shear stress.J Theor Biol. 2005 Feb 21;232(4):569-85. doi: 10.1016/j.jtbi.2004.09.004. J Theor Biol. 2005. PMID: 15588637
-
Assembly and mechanosensory function of focal contacts.Curr Opin Cell Biol. 2001 Oct;13(5):584-92. doi: 10.1016/s0955-0674(00)00255-6. Curr Opin Cell Biol. 2001. PMID: 11544027 Review.
-
Assembly and mechanosensory function of focal adhesions: experiments and models.Eur J Cell Biol. 2006 Apr;85(3-4):165-73. doi: 10.1016/j.ejcb.2005.11.001. Epub 2005 Dec 19. Eur J Cell Biol. 2006. PMID: 16360240 Review.
Cited by
-
Systems mechanobiology: tension-inhibited protein turnover is sufficient to physically control gene circuits.Biophys J. 2014 Dec 2;107(11):2734-43. doi: 10.1016/j.bpj.2014.10.042. Epub 2014 Dec 2. Biophys J. 2014. PMID: 25468352 Free PMC article.
-
Membrane related dynamics and the formation of actin in cells growing on micro-topographies: a spatial computational model.BMC Syst Biol. 2014 Sep 9;8:106. doi: 10.1186/s12918-014-0106-2. BMC Syst Biol. 2014. PMID: 25200251 Free PMC article.
-
Systems perspective on mechanobiology: producing the right proteins in the right place at the right time.Biophys J. 2014 Dec 2;107(11):2490-1. doi: 10.1016/j.bpj.2014.10.043. Epub 2014 Dec 2. Biophys J. 2014. PMID: 25468327 Free PMC article. No abstract available.
-
Coordinated Expression of Ras Suppressor 1 (RSU-1) and Growth Differentiation Factor 15 (GDF15) Affects Glioma Cell Invasion.Cancers (Basel). 2019 Aug 13;11(8):1159. doi: 10.3390/cancers11081159. Cancers (Basel). 2019. PMID: 31412547 Free PMC article.
-
PAK4 methylation by the methyltransferase SETD6 attenuates cell adhesion.Sci Rep. 2020 Oct 13;10(1):17068. doi: 10.1038/s41598-020-74081-1. Sci Rep. 2020. PMID: 33051544 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

