Melatonin suppresses cisplatin-induced nephrotoxicity via activation of Nrf-2/HO-1 pathway
- PMID: 23311701
- PMCID: PMC3561216
- DOI: 10.1186/1743-7075-10-7
Melatonin suppresses cisplatin-induced nephrotoxicity via activation of Nrf-2/HO-1 pathway
Abstract
Background: Cisplatin, one of the most effective and potent anticancer drugs, is used in the treatment of a wide variety of both pediatric and adult malignancies. However, the chemotherapeutic use of cisplatin is limited by its serious side-effects such as nephrotoxicity and ototoxicity. Cisplatin chemotherapy induces a reduction in the antioxidant status, leading to a failure of the antioxidant defense against free-radical damage generated by antitumor drugs. Cisplatin-induced oxidative stress in the kidney was partially prevented by antioxidant treatments using superoxide dismutase, glutathione, selenium and flavonoids. Melatonin and its metabolites possess free-radical scavenging activity and it has been shown that they protect against cisplatin toxicity. However, the mechanism of the protective effects of melatonin against cisplatin-induced nephrotoxicity is still essentially unknown. We therefore designed this study to investigate the underlying mechanism of the protective effect of melatonin against cisplatin-induced renal damage in a rat nephrotoxicity model in vivo.
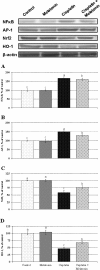
Methods: Twenty eight 8-week-old male Wistar rats were divided into four groups of control, melatonin treatment (4 mg/kg b.w i.p. for 10 days), cisplatin treatment (7 mg/kg b.w., i.p.) and melatonin and cisplatin combination treatment. Serum urea nitrogen (urea-N) and creatinine levels were measured. Histopathological changes were evaluated. In addition, we analyzed the expression levels of HO-1, Nrf2, NF-κB and AP-1 in Western blot analysis.
Results: Both serum creatinine and urea nitrogen increased significantly following cisplatin administration alone; these values decreased significantly with melatonin co-treatment of cisplatin-treated rats. Histological analysis showed that cisplatin caused damage in the proximal tubular cells in the kidneys of cisplatin-treated rats; these changes were reversed by melatonin co-treatment. Upon Western blot analysis, melatonin treatment increased Nrf2 accumulation in the nuclear fraction, and increased the expression of HO-1 in the cytosolic fraction as compared to the cisplatin-treated rats. Expressions of NF-κB p65 and AP-1 were increased significantly in the kidneys of rats treated with cisplatin compared with the expression in the kidneys from the control, melatonin-only-treated and melatonin co-treated rats.
Conclusion: Our present data suggest that melatonin attenuates cisplatin-induced nephrotoxicity possibly by modulating Nrf2/HO-1 signaling.
Figures

References
-
- Safirstein R, Winston J, Goldstein M, Moel D, Dikman S, Guttenplan J. Cisplatin nephrotoxicity. Am J Kidney Dis. 1986;8:356–367. - PubMed
-
- Osanto S, Bukman A, Van Hoek F, Sterk PJ, De Laat JA, Hermans J. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol. 1992;10:574–579. - PubMed
-
- Luke DR, Vadiei K, Lopez-Berestein G. Role of vascular congestion in cisplatin-induced acute renal failure in the rat. Nephrol Dial Transplant. 1992;37(1):1–7. - PubMed
-
- Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001;12:2683–2690. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

