Identification of Michael acceptor-centric pharmacophores with substituents that yield strong thioredoxin reductase inhibitory character correlated to antiproliferative activity
- PMID: 23311917
- PMCID: PMC3786391
- DOI: 10.1089/ars.2012.4909
Identification of Michael acceptor-centric pharmacophores with substituents that yield strong thioredoxin reductase inhibitory character correlated to antiproliferative activity
Abstract
Aims: The role of thioredoxin reductase (TrxR) in tumorigenesis has made it an attractive anticancer target. A systematic approach for development of novel compounds as TrxR inhibitors is currently lacking. Structurally diversified TrxR inhibitors share in common electrophilic propensities for the sulfhydryl groups, among which include the Michael reaction acceptors containing an α,β-unsaturated carbonyl moiety. We aimed to identify features among structurally diversified Michael acceptor-based compounds that would yield a strong TrxR inhibitory character.
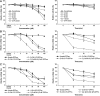
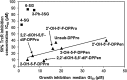
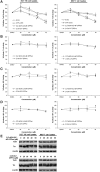
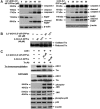
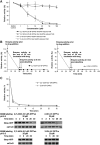
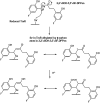
Results: Structurally dissimilar Michael acceptor-based natural compounds such as isobutylamides, zerumbone, and shogaols (SGs) were found to possess a poor TrxR inhibitory activity, indicating that a sole Michael acceptor moiety was insufficient to produce TrxR inhibition. The 1,7-diphenyl-hept-3-en-5-one pharmacophore in 3-phenyl-3-SG, a novel SG analog that possessed comparable TrxR inhibitory and antiproliferative potencies as 6-SG, was modified to yield 1,5-diphenyl-pent-1-en-3-one (DPPen) and 1,3-diphenyl-pro-1-en-3-one (DPPro, also known as chalcone) pharmacophores. These Michael acceptor-centric pharmacophores, when substituted with the hydroxyl and fluorine groups, gave rise to analogs displaying a TrxR inhibitory character positively correlated to their antiproliferative potencies. Lead analogs 2,2'-diOH-5,5'-diF-DPPen and 2-OH-5-F-DPPro yielded a half-maximal TrxR inhibitory concentration of 9.1 and 10.5 μM, respectively, after 1-h incubation with recombinant rat TrxR, with the C-terminal selenocysteine residue found to be targeted.
Innovation: Identification of Michael acceptor-centric pharmacophores among diversified compounds demonstrates that a systematic approach to discover and develop Michael acceptor-based TrxR inhibitors is feasible.
Conclusion: A strong TrxR inhibitory character correlated to the antiproliferative potency is attributed to structural features that include an α,β-unsaturated carbonyl moiety centered in a DPPen or DPPro pharmacophore bearing hydroxyl and fluorine substitutions.
Figures
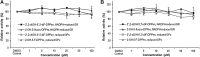

Similar articles
-
Indolin-2-one compounds targeting thioredoxin reductase as potential anticancer drug leads.Oncotarget. 2016 Jun 28;7(26):40233-40251. doi: 10.18632/oncotarget.9579. Oncotarget. 2016. PMID: 27244886 Free PMC article.
-
Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: potential candidates for cancer therapy and chemoprevention.Free Radic Biol Med. 2010 Jan 1;48(1):98-111. doi: 10.1016/j.freeradbiomed.2009.10.028. Epub 2009 Oct 27. Free Radic Biol Med. 2010. PMID: 19837157
-
Thioredoxin reductase inhibition by antitumor quinols: a quinol pharmacophore effect correlating to antiproliferative activity.FASEB J. 2008 Jun;22(6):2072-83. doi: 10.1096/fj.07-101477. Epub 2008 Jan 7. FASEB J. 2008. PMID: 18180330
-
Thioredoxin reductase inhibitors: a patent review.Expert Opin Ther Pat. 2017 May;27(5):547-556. doi: 10.1080/13543776.2017.1272576. Epub 2016 Dec 26. Expert Opin Ther Pat. 2017. PMID: 27977313 Review.
-
Thioredoxin reductase inhibitors: updated patent review (2017-present).Expert Opin Ther Pat. 2021 Aug;31(8):745-758. doi: 10.1080/13543776.2021.1899160. Epub 2021 Mar 22. Expert Opin Ther Pat. 2021. PMID: 33666133 Review.
Cited by
-
ROS generation mediates the anti-cancer effects of WZ35 via activating JNK and ER stress apoptotic pathways in gastric cancer.Oncotarget. 2015 Mar 20;6(8):5860-76. doi: 10.18632/oncotarget.3333. Oncotarget. 2015. PMID: 25714022 Free PMC article.
-
Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes.Pharmaceuticals (Basel). 2021 Jun 15;14(6):571. doi: 10.3390/ph14060571. Pharmaceuticals (Basel). 2021. PMID: 34203813 Free PMC article. Review.
-
Brevetoxin-2, is a unique inhibitor of the C-terminal redox center of mammalian thioredoxin reductase-1.Toxicol Appl Pharmacol. 2017 Aug 15;329:58-66. doi: 10.1016/j.taap.2017.05.027. Epub 2017 May 25. Toxicol Appl Pharmacol. 2017. PMID: 28551108 Free PMC article.
-
Bioactive Clerodane Diterpenoids from the Leaves of Casearia coriacea Vent.Molecules. 2023 Jan 25;28(3):1197. doi: 10.3390/molecules28031197. Molecules. 2023. PMID: 36770864 Free PMC article.
-
Cellular Protection of SNAP-25 against Botulinum Neurotoxin/A: Inhibition of Thioredoxin Reductase through a Suicide Substrate Mechanism.J Am Chem Soc. 2016 May 4;138(17):5568-75. doi: 10.1021/jacs.5b12929. Epub 2016 Apr 20. J Am Chem Soc. 2016. PMID: 27070533 Free PMC article.
References
-
- Arnér ES. Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. - PubMed
-
- Arnér ES. Sarioglu H. Lottspeich F. Holmgren A. Böck A. High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes. J Mol Biol. 1999;292:1003–1016. - PubMed
-
- Arscott LD. Gromer S. Schirmer RH. Becker K. Williams CH., Jr The mechanism of thioredoxin reductase from human placenta is similar to the mechanisms of lipoamide dehydrogenase and glutathione reductase and is distinct from the mechanism of thioredoxin reductase from Escherichia coli. Proc Natl Acad Sci U S A. 1997;94:3621–3626. - PMC - PubMed
-
- Becker K. Herold-Mende C. Park JJ. Lowe G. Schirmer RH. Human thioredoxin reductase is efficiently inhibited by (2,2′:6′,2′ ′-terpyridine)platinum(II) complexes. Possible implications for a novel antitumor strategy. J Med Chem. 2001;44:2784–2792. - PubMed
-
- Berggren M. Gallegos A. Gasdaska JR. Gasdaska PY. Warneke J. Powis G. Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res. 1996;16:3459–3466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

