Influence of N-terminal residue composition on the structure of proline-containing b2+ ions
- PMID: 23312013
- PMCID: PMC3641857
- DOI: 10.1021/jp306759f
Influence of N-terminal residue composition on the structure of proline-containing b2+ ions
Abstract
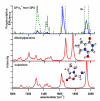
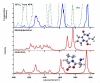
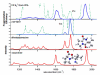
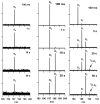
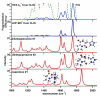
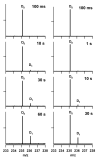
To probe the structural implications of the proline residue on its characteristic peptide fragmentation patterns, in particular its unusual cleavage at its C-terminus in formation of a b(2) ion in XxxProZzz sequences, the structures of a series of proline-containing b(2)(+) ions were studied by using action infrared multiphoton dissociation (IRMPD) spectroscopy and fragment ion hydrogen-deuterium exchange (HDX). Five different Xxx-Pro b(2)(+) ions were studied, with glycine, alanine, isoleucine, valine, or histidine in the N-terminal position. The residues selected feature different sizes, chain lengths, and gas phase basicities to explore whether the structure of the N-terminal residue influences the Xxx-Pro b(2)(+) ion structure. In proteins, the proline side chain-to-backbone attachment causes its peptide bonds to be in the cis conformation more than any other amino acid, although trans is still favored over cis. However, HP is the only b(2)(+) ion studied here that forms the diketopiperazine exclusively. The GP, AP, IP, and VP b(2)(+) ions formed from protonated tripeptide precursors predominantly featured oxazolone structures with small diketopiperazine contributions. In contrast to the b(2)(+) ions generated from tripeptides, synthetic cyclic dipeptides VP and HP were confirmed to have exclusive diketopiperazine structures.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

