Fatty acid-mediated endoplasmic reticulum stress in vivo: differential response to the infusion of Soybean and Lard Oil in rats
- PMID: 23312405
- PMCID: PMC3633667
- DOI: 10.1016/j.metabol.2012.12.001
Fatty acid-mediated endoplasmic reticulum stress in vivo: differential response to the infusion of Soybean and Lard Oil in rats
Abstract
Background: In cell systems, saturated fatty acids, compared to unsaturated fatty acids, induce a greater degree of ER stress and inflammatory signaling in a number of cell types, including hepatocytes and adipocytes. The aim of the present study was to determine the effects of infusions of lard oil (enriched in saturated fatty acids) and soybean oil (enriched in unsaturated fatty acids) on liver and adipose tissue ER stress and inflammatory signaling in vivo.
Methods: Lipid emulsions containing glycerol, phosphatidylcholine, antibiotics (Control, n=7) and either soybean oil (Soybean, n=7) or lard oil (Lard, n=7) were infused intravenously into rats over a 4 h period.
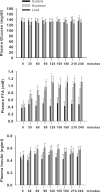
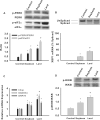
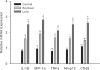
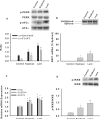
Results: Plasma free fatty acid levels were 0.5±0.1 mmol/L (mean±SD) in Control and were increased to 1.0±0.3 mmol/L and 1.1±0.3 mmol/L in Soybean and Lard, respectively. Glucose and insulin levels were not different among groups. Markers of endoplasmic reticulum (ER) stress and activation of inflammatory pathway signaling were increased in liver and adipose tissue from Soybean and Lard compared to Control, but were increased to a greater extent in Lard compared to Soybean.
Conclusions: These data suggest that elevated plasma free fatty acids can induce hepatic and adipose tissue ER stress and inflammation in vivo. In addition, saturated fatty acids appear to be more cytotoxic than unsaturated fatty acids in vivo.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Differential responses of hepatic endoplasmic reticulum stress and inflammation in diet-induced obese rats with high-fat diet rich in lard oil or soybean oil.PLoS One. 2013 Nov 6;8(11):e78620. doi: 10.1371/journal.pone.0078620. eCollection 2013. PLoS One. 2013. PMID: 24223162 Free PMC article.
-
In vivo effects of polyunsaturated, monounsaturated, and saturated fatty acids on hepatic and peripheral insulin sensitivity.Metabolism. 2015 Feb;64(2):315-22. doi: 10.1016/j.metabol.2014.10.019. Epub 2014 Oct 25. Metabolism. 2015. PMID: 25467844
-
The composition of dietary fat directly influences glucose-stimulated insulin secretion in rats.Diabetes. 2002 Jun;51(6):1825-33. doi: 10.2337/diabetes.51.6.1825. Diabetes. 2002. PMID: 12031970
-
Fatty acids, lipid emulsions and the immune and inflammatory systems.World Rev Nutr Diet. 2015;112:17-30. doi: 10.1159/000365426. Epub 2014 Nov 24. World Rev Nutr Diet. 2015. PMID: 25471799 Review.
-
Mechanisms of Action of trans Fatty Acids.Adv Nutr. 2020 May 1;11(3):697-708. doi: 10.1093/advances/nmz125. Adv Nutr. 2020. PMID: 31782488 Free PMC article. Review.
Cited by
-
Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States-Current View of Knowledge.Cells. 2022 Mar 1;11(5):844. doi: 10.3390/cells11050844. Cells. 2022. PMID: 35269467 Free PMC article. Review.
-
Endoplasmic reticulum membrane potassium channel dysfunction in high fat diet induced stress in rat hepatocytes.EXCLI J. 2014 Sep 9;13:1075-87. eCollection 2014. EXCLI J. 2014. PMID: 26417322 Free PMC article.
-
Lipotoxicity as a Barrier for T Cell-Based Therapies.Biomolecules. 2022 Aug 25;12(9):1182. doi: 10.3390/biom12091182. Biomolecules. 2022. PMID: 36139021 Free PMC article. Review.
-
Intravenous Lipid Emulsions in Parenteral Nutrition.Adv Nutr. 2015 Sep 15;6(5):600-10. doi: 10.3945/an.115.009084. Print 2015 Sep. Adv Nutr. 2015. PMID: 26374182 Free PMC article. Review.
-
The Influence of Obesity and Associated Fatty Acids on Placental Inflammation.Clin Ther. 2021 Feb;43(2):265-278. doi: 10.1016/j.clinthera.2020.12.018. Epub 2021 Jan 21. Clin Ther. 2021. PMID: 33487441 Free PMC article. Review.
References
-
- Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46(11):2347–2352. - PubMed
-
- Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29(5):478–485. - PubMed
-
- Rigazio S, Lehto H-R, Tuunanen H, Nagren K, Kankaanpaa M, Simi C, Borra R, Naum AG, Parkkola R, Knuuti J, et al. The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans. Am J Physiol Endocrinol Metab. 2008;295:E413–E419. - PubMed
-
- Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

