Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology
- PMID: 23312564
- PMCID: PMC3633722
- DOI: 10.1016/j.biopsych.2012.12.003
Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology
Abstract
Background: Increased circulating glucocorticoids are features of both aging and Alzheimer's disease (AD), and increased glucocorticoids accelerate the accumulation of AD pathologies. Here, we analyzed the effects of the glucocorticoid receptor antagonist mifepristone (RU486) in the 3xTg-AD mouse model at an age where hippocampal damage leads to high circulating corticosterone levels.
Methods: The effects of mifepristone were investigated in 3xTg-AD mice using a combination of biochemical, histological, and behavior analyses.
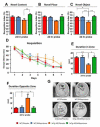
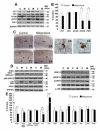
Results: Mifepristone treatment rescues the pathologically induced cognitive impairments and markedly reduces amyloid beta (Aβ)-load and levels, as well as tau pathologies. Analysis of amyloid precursor protein (APP) processing revealed concomitant decreases in both APP C-terminal fragments C99 and C83 and the appearance of a larger 17-kDa C-terminal fragment. Hence, mifepristone induces a novel C-terminal cleavage of APP that prevents it being cleaved by α- or β-secretase, thereby precluding Aβ generation in the central nervous system; this cleavage and the production of the 17-kDa APP fragment was generated by a calcium-dependent cysteine protease. In addition, mifepristone treatment also reduced the phosphorylation and accumulation of tau, concomitant with reductions in p25. Notably, deficits in cyclic-AMP response element-binding protein signaling were restored with the treatment.
Conclusions: These preclinical results point to a potential therapeutic role for mifepristone as an effective treatment for AD and further highlight the impact the glucocorticoid system has as a regulator of Aβ generation.
Keywords: 3xTg-AD; Alzheimer's disease; Amyloid beta; glucocorticoids; mifepristone; tau.
Copyright © 2013 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
It may take more than a shot: alternatives to immunotherapy for Alzheimer's disease.Biol Psychiatry. 2013 Sep 1;74(5):316-7. doi: 10.1016/j.biopsych.2013.07.003. Biol Psychiatry. 2013. PMID: 23932341 No abstract available.
References
-
- Hoogendijk WJ, Meynen G, Endert E, Hofman MA, Swaab DF. Increased cerebrospinal fluid cortisol level in Alzheimer’s disease is not related to depression. Neurobiol Aging. 2006;275:780 e781–780 e782. - PubMed
-
- Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, et al. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: a cross-sectional and longitudinal study. Brain Res. 2000;8812:241–243. - PubMed
-
- Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, et al. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry. 2009;141:95–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

