Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models
- PMID: 23312803
- PMCID: PMC3632303
- DOI: 10.1016/j.neurobiolaging.2012.12.005
Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models
Abstract
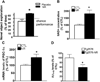
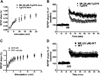
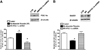
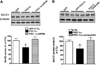
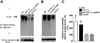
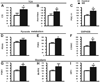
Nicotinamide adenine dinucleotide (NAD)(+), a coenzyme involved in redox activities in the mitochondrial electron transport chain, has been identified as a key regulator of the lifespan-extending effects, and the activation of NAD(+) expression has been linked with a decrease in beta-amyloid (Aβ) toxicity in Alzheimer's disease (AD). Nicotinamide riboside (NR) is a NAD(+) precursor, it promotes peroxisome proliferator-activated receptor-γ coactivator 1 (PGC)-1α expression in the brain. Evidence has shown that PGC-1α is a crucial regulator of Aβ generation because it affects β-secretase (BACE1) degradation. In this study we tested the hypothesis that NR treatment in an AD mouse model could attenuate Aβ toxicity through the activation of PGC-1α-mediated BACE1 degradation. Using the Tg2576 AD mouse model, using in vivo behavioral analyses, biochemistry assays, small hairpin RNA (shRNA) gene silencing and electrophysiological recording, we found (1) dietary treatment of Tg2576 mice with 250 mg/kg/day of NR for 3 months significantly attenuates cognitive deterioration in Tg2576 mice and coincides with an increase in the steady-state levels of NAD(+) in the cerebral cortex; (2) application of NR to hippocampal slices (10 μM) for 4 hours abolishes the deficits in long-term potentiation recorded in the CA1 region of Tg2576 mice; (3) NR treatment promotes PGC-1α expression in the brain coinciding with enhanced degradation of BACE1 and the reduction of Aβ production in Tg2576 mice. Further in vitro studies confirmed that BACE1 protein content is decreased by NR treatment in primary neuronal cultures derived from Tg2576 embryos, in which BACE1 degradation was prevented by PGC-1α-shRNA gene silencing; and (4) NR treatment and PGC-1α overexpression enhance BACE1 ubiquitination and proteasomal degradation. Our studies suggest that dietary treatment with NR might benefit AD cognitive function and synaptic plasticity, in part by promoting PGC-1α-mediated BACE1 ubiquitination and degradation, thus preventing Aβ production in the brain.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors have no potential conflicts of interest.
All experiments were approved by the Mount Sinai School of Medicine Animal Care committees.
Figures

Similar articles
-
SCFFbx2-E3-ligase-mediated degradation of BACE1 attenuates Alzheimer's disease amyloidosis and improves synaptic function.Aging Cell. 2010 Dec;9(6):1018-31. doi: 10.1111/j.1474-9726.2010.00632.x. Epub 2010 Oct 29. Aging Cell. 2010. PMID: 20854419 Free PMC article.
-
PPARγ-coactivator-1α gene transfer reduces neuronal loss and amyloid-β generation by reducing β-secretase in an Alzheimer's disease model.Proc Natl Acad Sci U S A. 2016 Oct 25;113(43):12292-12297. doi: 10.1073/pnas.1606171113. Epub 2016 Oct 10. Proc Natl Acad Sci U S A. 2016. PMID: 27791018 Free PMC article.
-
Can nicotinamide riboside protect against cognitive impairment?Curr Opin Clin Nutr Metab Care. 2020 Nov;23(6):413-420. doi: 10.1097/MCO.0000000000000691. Curr Opin Clin Nutr Metab Care. 2020. PMID: 32925178 Review.
-
PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia.Arch Neurol. 2009 Mar;66(3):352-61. doi: 10.1001/archneurol.2008.588. Arch Neurol. 2009. PMID: 19273754 Free PMC article.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
-
Cystatin C Shifts APP Processing from Amyloid-β Production towards Non-Amyloidgenic Pathway in Brain Endothelial Cells.PLoS One. 2016 Aug 17;11(8):e0161093. doi: 10.1371/journal.pone.0161093. eCollection 2016. PLoS One. 2016. PMID: 27532339 Free PMC article.
-
Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders.Clin Transl Med. 2016 Dec;5(1):25. doi: 10.1186/s40169-016-0104-7. Epub 2016 Jul 27. Clin Transl Med. 2016. PMID: 27465020 Free PMC article. Review.
-
Alzheimer's disease pathology is attenuated in a CD38-deficient mouse model.Ann Neurol. 2015 Jul;78(1):88-103. doi: 10.1002/ana.24425. Epub 2015 May 25. Ann Neurol. 2015. PMID: 25893674 Free PMC article.
-
Nicotinamide Mononucleotide Supplementation Improves Mitochondrial Dysfunction and Rescues Cellular Senescence by NAD+/Sirt3 Pathway in Mesenchymal Stem Cells.Int J Mol Sci. 2022 Nov 25;23(23):14739. doi: 10.3390/ijms232314739. Int J Mol Sci. 2022. PMID: 36499074 Free PMC article.
-
Mitophagy and Neurodegeneration: Between the Knowns and the Unknowns.Front Cell Dev Biol. 2022 Mar 22;10:837337. doi: 10.3389/fcell.2022.837337. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35392168 Free PMC article. Review.
References
-
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide Riboside Promotes Sir2 Silencing and Extends Lifespan via Nrk and Urh1/Pnp1/Meu1 Pathways to NAD+ Cell. 2007;129:473–484. - PubMed
-
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 2006;1:1306–1311. - PubMed
-
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. - PubMed
-
- Bieganowski P, Pace HC, Brenner C. Eukaryotic NAD+ synthetase Qns1 contains an essential, obligate intramolecular thiol glutamine amidotransferase domain related to nitrilase. J. Biol. Chem. 2003;278:33049–33055. - PubMed
-
- Braidy N, Guillemin G, Grant R. Promotion of cellular NAD(+) anabolism: therapeutic potential for oxidative stress in ageing and Alzheimer’s disease. Neurotox. Res. 2008;13:173–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

