Contraceptive vaginal ring effectiveness is maintained during 6 weeks of use: a prospective study of normal BMI and obese women
- PMID: 23312933
- PMCID: PMC3606661
- DOI: 10.1016/j.contraception.2012.12.001
Contraceptive vaginal ring effectiveness is maintained during 6 weeks of use: a prospective study of normal BMI and obese women
Abstract
Background: A single study shows that contraceptive vaginal ring (CVR) use for up to 35 days in women with a normal body mass index (BMI) maintains serum hormone levels sufficient to suppress ovulation. This study is intended to confirm those results and to evaluate prolonged CVR use up to 42 days in both normal BMI and obese women.
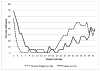
Study design: Twenty women with a normal BMI and 20 obese women enrolled in a prospective open label clinical study of ethinyl estradiol (EE) and etonogestrel (ENG) pharmacokinetics during six weeks of use of a single CVR. Participants underwent twice-weekly evaluations to determine serum hormone concentrations, ovarian follicle development, endometrial thickness and bleeding patterns.
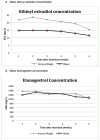
Results: Thirty-seven women completed follow-up including eighteen women with a normal BMI and nineteen obese women. EE and ENG concentrations remained in therapeutic range for all women. Follicular development and endometrial proliferation were minimal. By the sixth week, 30% of participants reported spotting or bleeding.
Conclusions: A single CVR used for 6 weeks demonstrates therapeutic serum levels of EE and ENG among women with normal and obese BMI. Women who forget to remove the CVR at day 21 may well have continued contraceptive protection during the next 3 weeks.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Pharmacokinetics and ovarian suppression during use of a contraceptive vaginal ring in normal-weight and obese women.Am J Obstet Gynecol. 2012 Jul;207(1):39.e1-6. doi: 10.1016/j.ajog.2012.04.022. Epub 2012 Apr 28. Am J Obstet Gynecol. 2012. PMID: 22727346 Free PMC article. Clinical Trial.
-
A comparative study of two contraceptive vaginal rings releasing norethindrone acetate and differing doses of ethinyl estradiol.Contraception. 1999 May;59(5):305-10. doi: 10.1016/s0010-7824(99)00036-0. Contraception. 1999. PMID: 10494484 Clinical Trial.
-
Efficacy, bleeding patterns, and side effects of a 1-year contraceptive vaginal ring.Contraception. 1999 May;59(5):311-8. doi: 10.1016/s0010-7824(99)00035-9. Contraception. 1999. PMID: 10494485
-
Complete and robust ovulation inhibition with NuvaRing.Eur J Contracept Reprod Health Care. 2002 Dec;7 Suppl 2:13-8; discussion 37-9. Eur J Contracept Reprod Health Care. 2002. PMID: 12659397 Review.
-
The combined etonogestrel/ethinyl estradiol contraceptive vaginal ring.Expert Opin Pharmacother. 2007 Aug;8(11):1769-77. doi: 10.1517/14656566.8.11.1769. Expert Opin Pharmacother. 2007. PMID: 17685892 Review.
References
-
- Nuvaring® [package insert] Roseland, NJ: Organon, a subsidiary of Merck & Co., Inc; 2001. p. 2010.
-
- Frost JJ, Darroch JE, Remez L. In Brief. Vol. 1 New York: Guttmacher Institute; 2008. Improving contraceptive use in the United States. - PubMed
-
- Ahrendt HJ, Nisand I, Bastianelli C, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 mcg of ethinyl estradiol and 3 mg of drospirenone. Contraception. 2006;74:451–7. - PubMed
-
- Oddsson K, Leifels-Fischer B, de Melo N, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005;71:176–82. - PubMed
-
- Roumen F, op ten Berg M, Hoomans E. The combined contraceptive vaginal ring (NuvaRing): first experience in daily clinical practice in The Netherlands. Eur J Contracept Reprod Health Care. 2006;11:14–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical