Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease
- PMID: 23312977
- PMCID: PMC4057430
- DOI: 10.1016/j.jtcvs.2012.12.027
Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease
Abstract
Background: Thoracic aortic aneurysms (TAAs) develop through an asymptomatic process resulting in gross dilation that progresses to rupture if left undetected and untreated. If detected, patients with TAA are followed over time until the risk of rupture outweighs the risk of surgical repair. Current methodologies for tracking TAA size are limited to expensive computed tomography or magnetic resonance imaging because no acceptable population screening tools are currently available. Previous studies from this laboratory and others have identified differential protein profiles for the matrix metalloproteinases (MMPs) and their endogenous tissue inhibitors (TIMPs), in ascending TAA tissue from patients with bicuspid aortic valves (BAVs), versus patients with idiopathic degenerative disease and a tricuspid aortic valve (TAV). In addition, altered microRNA (miR) expression levels have also been reported in TAAs compared with normal aortic tissue. The objective of our study was to identify circulating factors within plasma that could serve as potential biomarkers for distinguishing etiologic subtypes of aneurysm disease.
Methods: Ascending TAA tissue and plasma specimens were obtained from patients with BAV (n = 21) and TAV (n = 21) at the time of surgical resection. The protein abundance of key MMPs (1, 2, 3, 8, and 9), TIMPs (1, 2, 3, and 4), and miRs (1, 21, 29a, 133a, 143, and 145) was examined using a multianalyte protein profiling system or by quantitative polymerase chain reaction, respectively. Results were compared with normal aortic tissue and plasma obtained from patients without aortic disease (n = 10).
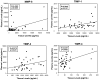
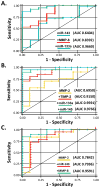
Results: Significant (P < .05) differences in standardized miR-1 and miR-21 abundance between BAV and TAV aortic tissue samples and different tissue and plasma profiles of analyte differences from normal aorta where observed between the BAV and TAV groups. Linear regression analysis revealed significant linear relationships in plasma and tissue measurements only for MMP-8 and TIMP-1, TIMP-3, and TIMP-4 (P < .05). Receiver operator curve analysis revealed specific cassettes of analytes predictive of TAA disease. Relative to normal aorta, BAV proteolytic balance was significantly increased for MMP-1, MMP-2, and MMP-7, and for decreased MMP-8 and MMP-9. In contrast, TAV proteolytic balance relative to normal aorta was significantly increased only for MMP-1 and decreased for MMP-8 and MMP-9.
Conclusions: Taken together, these unique data demonstrate differential plasma profiles of MMPs, TIMPs, and miRs in ascending TAA specimens from patients with BAV and TAV. These results suggest that circulating biomarkers may form the foundation for a broader platform of biomarkers capable of detecting the presence of TAA using a simple blood test and may also be useful in personalized strategies to distinguish between etiologic subtypes of TAAs in patients with aneurysm disease.
Copyright © 2013 The American Association for Thoracic Surgery. Published by Mosby, Inc. All rights reserved.
Figures

Comment in
-
The molecular fingerprint of bicuspid aortopathy.J Thorac Cardiovasc Surg. 2013 May;145(5):1334. doi: 10.1016/j.jtcvs.2013.02.067. J Thorac Cardiovasc Surg. 2013. PMID: 23597625 No abstract available.
References
-
- Fedak PW, de Sa MP, Verma S, et al. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg. 2003;126:797–806. - PubMed
-
- LeMaire SA, Wang X, Wilks JA, et al. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005;123:40–8. - PubMed
-
- Ikonomidis JS, Jones JA, Barbour JR, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114:I365–70. - PubMed
-
- Ikonomidis JS, Jones JA, Barbour JR, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133:1028–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

