AP301, a synthetic peptide mimicking the lectin-like domain of TNF, enhances amiloride-sensitive Na(+) current in primary dog, pig and rat alveolar type II cells
- PMID: 23313096
- PMCID: PMC3646188
- DOI: 10.1016/j.pupt.2012.12.011
AP301, a synthetic peptide mimicking the lectin-like domain of TNF, enhances amiloride-sensitive Na(+) current in primary dog, pig and rat alveolar type II cells
Abstract
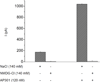
Pulmonary permeability oedema is a frequent complication in a number of life-threatening lung conditions, such as ALI and ARDS. Apart from ventilation strategies, no specific therapy yet exists for treatment of these potentially fatal illnesses. The oedema-reducing capacity of the lectin-like domain of TNF (TIP) and of synthetic peptides, mTIP and hTIP, which mimic the TIP domain of mouse and human TNF, have been demonstrated in various studies in rodents. Cell-based electrophysiological studies have revealed that the alveolar fluid clearing capacity of TNF and the TIP peptides is due to activation of the amiloride-sensitive Na(+) current in alveolar epithelial cells and that the primary site of action is on the apical side of these cells. AP301, a synthetic cyclic peptide mimicking the TIP domain of human TNF is currently undergoing clinical trials as a therapy for pulmonary permeability oedema. AP301 has been shown to improve alveolar liquid clearance and lung function in a porcine model of ALI. For non-clinical regulatory assessment, dog, pig and rat are standard animal models; accordingly, pre-clinical toxicological and pharmacological safety studies have been conducted with AP301 in dogs and rats. Hitherto, no studies have assessed the pharmacodynamic effect of AP301 on primary canine or porcine type II AEC. The current study describes the effect of AP301 on the amiloride-sensitive Na(+) current in type II AEC isolated from dog, pig and rat lungs. In whole cell patch clamp experiments with dog type II AEC, an increase in the amiloride-sensitive Na(+) current from 3.7 pA to 49.4 pA was observed in the presence of AP301; in pig type II AEC, an increase from 10.0 pA to 159.6 pA was observed, and in rat AEC, from 6.9 pA to 62.4 pA. In whole cell patch clamp experiments in A549 cells, AP301-induced enhancement of the amiloride-sensitive current was eliminated when Na(+) in the bath solution was replaced with N-methyl-d-glucamine (NMDG), and when the cells were pre-incubated with 5-aminoimidazole-4-carboxamide-1-ß-d-ribofuranoside (AICAR), an inhibitor of ENaC, but enhancement was unaffected by addition of cyclic nucleotide-gated (CNG) channel inhibitors Zn(2+) or l-cis-diltiazem prior to AP301. These results provide strong evidence that AP301 activates the amiloride-sensitive Na(+) current through ENaC in type II AEC from dog, pig and rat. To our knowledge, this is the first cell-based analysis of the oedema-clearing effect of AP301 observed in the porcine model of pulmonary oedema. Furthermore, the results validate the dog and pig models in non-clinical assessment of AP301.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures






References
-
- Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2001;163:1376–1383. - PubMed
-
- Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annual review of physiology. 2009;71:403–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

