Isozyme profile and tissue-origin of alkaline phosphatases in mouse serum
- PMID: 23313280
- PMCID: PMC3593980
- DOI: 10.1016/j.bone.2012.12.048
Isozyme profile and tissue-origin of alkaline phosphatases in mouse serum
Abstract
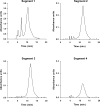
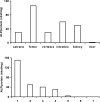
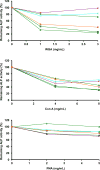
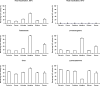
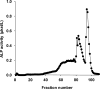
Mouse serum alkaline phosphatase (ALP) is frequently measured and interpreted in mammalian bone research. However, little is known about the circulating ALPs in mice and their relation to human ALP isozymes and isoforms. Mouse ALP was extracted from liver, kidney, intestine, and bone from vertebra, femur and calvaria tissues. Serum from mixed strains of wild-type (WT) mice and from individual ALP knockout strains were investigated, i.e., Alpl(-/-) (a.k.a. Akp2 encoding tissue-nonspecific ALP or TNALP), Akp3(-/-) (encoding duodenum-specific intestinal ALP or dIALP), and Alpi(-/-) (a.k.a. Akp6 encoding global intestinal ALP or gIALP). The ALP isozymes and isoforms were identified by various techniques and quantified by high-performance liquid chromatography. Results from the WT and knockout mouse models revealed identical bone-specific ALP isoforms (B/I, B1, and B2) as found in human serum, but in addition mouse serum contains the B1x isoform only detected earlier in patients with chronic kidney disease and in human bone tissue. The two murine intestinal isozymes, dIALP and gIALP, were also identified in mouse serum. All four bone-specific ALP isoforms (B/I, B1x, B1, and B2) were identified in mouse bones, in good correspondence with those found in human bones. All mouse tissues, except liver and colon, contained significant ALP activities. This is a notable difference as human liver contains vast amounts of ALP. Histochemical staining, Northern and Western blot analyses confirmed undetectable ALP expression in liver tissue. ALP activity staining showed some positive staining in the bile canaliculi for BALB/c and FVB/N WT mice, but not in C57Bl/6 and ICR mice. Taken together, while the main source of ALP in human serum originates from bone and liver, and a small fraction from intestine (<5%), mouse serum consists mostly of bone ALP, including all four isoforms, B/I, B1x, B1, and B2, and two intestinal ALP isozymes dIALP and gIALP. We suggest that the genetic nomenclature for the Alpl gene in mice (i.e., ALP liver) should be reconsidered since murine liver has undetectable amounts of ALP activity. These findings should pave the way for the development of user-friendly assays measuring circulating bone-specific ALP in mouse models used in bone and mineral research.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures



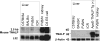
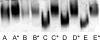
References
-
- McComb RB, Bowers GN, Jr, Posen S. Alkaline phosphatase. New York, NY, USA: Plenum Press; 1979.
-
- Millán JL. From biology to applications in medicine and biotechnology. Weinheim, Germany: WILEY-VCH Verlag GmgH & Co. KGaA; 2006. Mammalian alkaline phosphatase.
-
- Alatalo SL, Peng Z, Janckila AJ, Kaija H, Vihko P, Väänänen HK, et al. A novel immunoassay for the determination of tartrate-resistant acid phosphatase 5b from rat serum. J Bone Miner Res. 2003;18:134–9. - PubMed
-
- Srivastava AK, Bhattacharyya S, Castillo G, Miyakoshi N, Mohan S, Baylink DJ. Development and evaluation of C-telopeptide enzyme-linked immunoassay for measurement of bone resorption in mouse serum. Bone. 2000;27:529–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

