Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype
- PMID: 23313316
- PMCID: PMC3629000
- DOI: 10.1016/j.nbd.2012.12.016
Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype
Abstract
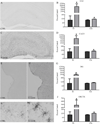
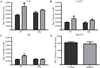
Excessive alcohol intake, a defining characteristic of an alcohol use disorder (AUD), results in neurodegeneration in the hippocampus and entorhinal cortex that has been linked to a variety of cognitive deficits. Neuroinflammation is thought to be a factor in alcohol-induced neurodegeneration, and microglia activation is a key but not sole component of an inflammatory response. These experiments investigate the effects of ethanol exposure in a well-accepted model of an AUD on both microglial activation and blood brain barrier disruption (BBB) in order to understand their relationship to classical definitions of inflammation and alcohol-induced neurodegeneration. Following a four-day binge ethanol paradigm, rat hippocampal and entorhinal cortex tissue was examined using three distinct approaches to determine microglia phenotype and BBB disruption: immunohistochemistry, autoradiography, and ELISA. After ethanol exposure, there was an increase in [(3)H]-PK-11195 binding and OX-42 immunoreactivity indicative of microglial activation; however, microglia were not fully activated since both OX-6 and ED-1 immunoreactive microglia were absent. This data was supported by functional evidence as there was no increase in the proinflammatory cytokines IL-6 or TNF-α, but a 26% increase in the anti-inflammatory cytokine, IL-10, and a 38% increase in the growth factor, TGF-β, seven days after exposure. Furthermore, there was no evidence of a disruption of the BBB. These data suggest that the four-day binge model of an AUD, which produces neurodegeneration in corticolimbic regions, does not elicit classical neuroinflammation but instead produces partially activated microglia. Partial activation of microglia following binge ethanol exposure suggest that microglia in this model have beneficial or homeostatic roles rather than directly contributing to neurodegeneration and are a consequence of alcohol-induced-damage instead of the source of damage.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

