Astrocyte GRK2 as a novel regulator of glutamate transport and brain damage
- PMID: 23313319
- PMCID: PMC3628971
- DOI: 10.1016/j.nbd.2012.12.013
Astrocyte GRK2 as a novel regulator of glutamate transport and brain damage
Abstract
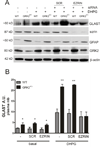
G protein-coupled receptor (GPCR) kinase 2 (GRK2) regulates cellular signaling via desensitization of GPCRs and by direct interaction with intracellular signaling molecules. We recently described that ischemic brain injury decreases cerebral GRK2 levels. Here we studied the effect of astrocyte GRK2-deficiency on neonatal brain damage in vivo. As astrocytes protect neurons by taking up glutamate via plasma-membrane transporters, we also studied the effect of GRK2 on the localization of the GLutamate ASpartate Transporter (GLAST). Brain damage induced by hypoxia-ischemia was significantly reduced in GFAP-GRK2(+/-) mice, which have a 60% reduction in astrocyte GRK2 compared to GFAP-WT littermates. In addition, GRK2-deficient astrocytes have higher plasma-membrane levels of GLAST and an increased capacity to take up glutamate in vitro. In search for the mechanism by which GRK2 regulates GLAST expression, we observed increased GFAP levels in GRK2-deficient astrocytes. GFAP and the cytoskeletal protein ezrin are known regulators of GLAST localization. In line with this evidence, GRK2-deficiency reduced phosphorylation of the GRK2 substrate ezrin and enforced plasma-membrane GLAST association after stimulation with the group I mGluR-agonist DHPG. When ezrin was silenced, the enhanced plasma-membrane GLAST association in DHPG-exposed GRK2-deficient astrocytes was prevented. In conclusion, we identified a novel role of astrocyte GRK2 in regulating plasma-membrane GLAST localization via an ezrin-dependent route. We demonstrate that the 60% reduction in astrocyte GRK2 protein level that is observed in GFAP-GRK2(+/-) mice is sufficient to significantly reduce neonatal ischemic brain damage. These findings underline the critical role of GRK2 regulation in astrocytes for dampening the extent of brain damage after ischemia.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Perspectives for Ezrin and Radixin in Astrocytes: Kinases, Functions and Pathology.Int J Mol Sci. 2019 Aug 2;20(15):3776. doi: 10.3390/ijms20153776. Int J Mol Sci. 2019. PMID: 31382374 Free PMC article. Review.
-
Low endogenous G-protein-coupled receptor kinase 2 sensitizes the immature brain to hypoxia-ischemia-induced gray and white matter damage.J Neurosci. 2008 Mar 26;28(13):3324-32. doi: 10.1523/JNEUROSCI.4769-07.2008. J Neurosci. 2008. PMID: 18367599 Free PMC article.
-
Differential regulation of GLAST immunoreactivity and activity by protein kinase C: evidence for modification of amino and carboxyl termini.J Neurochem. 2004 Dec;91(5):1151-63. doi: 10.1111/j.1471-4159.2004.02791.x. J Neurochem. 2004. PMID: 15569258
-
Thrombin decreases expression of the glutamate transporter GLAST and inhibits glutamate uptake in primary cortical astrocytes via the Rho kinase pathway.Exp Neurol. 2015 Nov;273:288-300. doi: 10.1016/j.expneurol.2015.09.009. Epub 2015 Sep 21. Exp Neurol. 2015. PMID: 26391563
-
Astrocyte-derived growth factors and estrogen neuroprotection: role of transforming growth factor-α in estrogen-induced upregulation of glutamate transporters in astrocytes.Mol Cell Endocrinol. 2014 May 25;389(1-2):58-64. doi: 10.1016/j.mce.2014.01.010. Epub 2014 Jan 18. Mol Cell Endocrinol. 2014. PMID: 24447465 Free PMC article. Review.
Cited by
-
Prefrontal Cortex Cytosolic Proteome and Machine Learning-Based Predictors of Resilience toward Chronic Social Isolation in Rats.Int J Mol Sci. 2024 Mar 6;25(5):3026. doi: 10.3390/ijms25053026. Int J Mol Sci. 2024. PMID: 38474271 Free PMC article.
-
IL4-10 Fusion Protein Is a Novel Drug to Treat Persistent Inflammatory Pain.J Neurosci. 2016 Jul 13;36(28):7353-63. doi: 10.1523/JNEUROSCI.0092-16.2016. J Neurosci. 2016. PMID: 27413147 Free PMC article.
-
Perspectives for Ezrin and Radixin in Astrocytes: Kinases, Functions and Pathology.Int J Mol Sci. 2019 Aug 2;20(15):3776. doi: 10.3390/ijms20153776. Int J Mol Sci. 2019. PMID: 31382374 Free PMC article. Review.
-
Cyclic AMP response element-binding protein (CREB) transcription factor in astrocytic synaptic communication.Front Synaptic Neurosci. 2023 Jan 4;14:1059918. doi: 10.3389/fnsyn.2022.1059918. eCollection 2022. Front Synaptic Neurosci. 2023. PMID: 36685081 Free PMC article. Review.
-
Neuroinflammation in preterm babies and autism spectrum disorders.Pediatr Res. 2019 Jan;85(2):155-165. doi: 10.1038/s41390-018-0208-4. Epub 2018 Nov 16. Pediatr Res. 2019. PMID: 30446768 Review.
References
-
- Dale LB, Bhattacharya M, Anborgh PH, Murdoch B, Bhatia M, Nakanishi S, Ferguson SS. G protein-coupled receptor kinasemediated desensitization of metabotropic glutamate receptor 1A protects against cell death. J Biol Chem. 2000;275:38213–38220. - PubMed
-
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

