LRIG2 mutations cause urofacial syndrome
- PMID: 23313374
- PMCID: PMC3567269
- DOI: 10.1016/j.ajhg.2012.12.002
LRIG2 mutations cause urofacial syndrome
Abstract
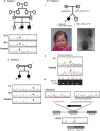
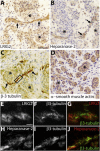
Urofacial syndrome (UFS) (or Ochoa syndrome) is an autosomal-recessive disease characterized by congenital urinary bladder dysfunction, associated with a significant risk of kidney failure, and an abnormal facial expression upon smiling, laughing, and crying. We report that a subset of UFS-affected individuals have biallelic mutations in LRIG2, encoding leucine-rich repeats and immunoglobulin-like domains 2, a protein implicated in neural cell signaling and tumorigenesis. Importantly, we have demonstrated that rare variants in LRIG2 might be relevant to nonsyndromic bladder disease. We have previously shown that UFS is also caused by mutations in HPSE2, encoding heparanase-2. LRIG2 and heparanase-2 were immunodetected in nerve fascicles growing between muscle bundles within the human fetal bladder, directly implicating both molecules in neural development in the lower urinary tract.
Copyright © 2013 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Kerecuk L., Schreuder M.F., Woolf A.S. Renal tract malformations: Perspectives for nephrologists. Nat. Clin. Pract. Nephrol. 2008;4:312–325. - PubMed
-
- Lambert H.J., Stewart A., Gullett A.M., Cordell H.J., Malcolm S., Feather S.A., Goodship J.A., Goodship T.H., Woolf A.S., UK VUR Study Group Primary, nonsyndromic vesicoureteric reflux and nephropathy in sibling pairs: A United Kingdom cohort for a DNA bank. Clin. J. Am. Soc. Nephrol. 2011;6:760–766. - PMC - PubMed
-
- Benarroch E.E. Neural control of the bladder: Recent advances and neurologic implications. Neurology. 2010;75:1839–1846. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

