Antigen sparing with adjuvanted inactivated polio vaccine based on Sabin strains
- PMID: 23313617
- PMCID: PMC3570672
- DOI: 10.1016/j.vaccine.2012.12.076
Antigen sparing with adjuvanted inactivated polio vaccine based on Sabin strains
Abstract
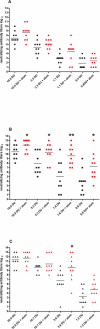
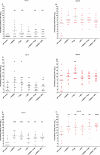
Six different adjuvants, each in combination with inactivated polio vaccine (IPV) produced with attenuated Sabin strains (sIPV), were evaluated for their ability to enhance virus neutralizing antibody titres (VNTs) in the rat potency model. The increase of VNTs was on average 3-, 15-, 24-fold with adjuvants after one immunization (serotypes 1, 2, and 3, respectively). Also after a boost immunization the VNTs of adjuvanted sIPV were on average another 7-20-27 times higher than after two inoculations of sIPV without adjuvant. The results indicate that it is feasible to increase the potency of inactivated polio vaccines by using adjuvants.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
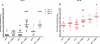
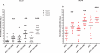
References
-
- Bakker WA, Thomassen YE, van't Oever AG, Westdijk J, van Oijen MG, Sundermann LC, et al. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk-IPV to Sabin-IPV. Vaccine. 2011 Sep 22;29(41):7188–96. - PubMed
-
- Westdijk J, Brugmans D, Martin J, van't Oever A, Bakker WA, Levels L, et al. Characterization and standardization of Sabin based inactivated polio vaccine: proposal for a new antigen unit for inactivated polio vaccines. Vaccine. 2011 Apr 18;29(18):3390–7. - PubMed
-
- Baldwin SL, Fox CB, Pallansch MA, Coler RN, Reed SG, Friede M. Increased potency of an inactivated trivalent polio vaccine with oil-in-water emulsions. Vaccine. 2011 Jan 17;29(4):644–9. - PubMed
-
- Ivanov AP, Dragunsky EM, Chumakov KM. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J Infect Dis. 2006 Feb 15;193(4):598–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

