Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study
- PMID: 23313811
- PMCID: PMC3888603
- DOI: 10.1136/annrheumdis-2012-202760
Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study
Abstract
Objective: To identify a suitable dosing regimen of the CD22-targeted monoclonal antibody epratuzumab in adults with moderately to severely active systemic lupus erythematosus (SLE).
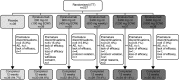
Methods: A phase IIb, multicentre, randomised controlled study (NCT00624351) was conducted with 227 patients (37-39 per arm) receiving either: placebo, epratuzumab 200 mg cumulative dose (cd) (100 mg every other week (EOW)), 800 mg cd (400 mg EOW), 2400 mg cd (600 mg weekly), 2400 mg cd (1200 mg EOW), or 3600 mg cd (1800 mg EOW). The primary endpoint (not powered for significance) was the week 12 responder rate measured using a novel composite endpoint, the British Isles Lupus Assessment Group (BILAG)-based Combined Lupus Assessment (BICLA).
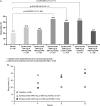
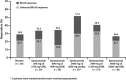
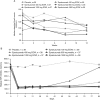
Results: Proportion of responders was higher in all epratuzumab groups than with placebo (overall treatment effect test p=0.148). Exploratory pairwise analysis demonstrated clinical improvement in patients receiving a cd of 2400 mg epratuzumab (OR for 600 mg weekly vs placebo: 3.2 (95% CI 1.1 to 8.8), nominal p=0.03; OR for 1200 mg EOW vs placebo: 2.6 (0.9 to 7.1), nominal p=0.07). Post-hoc comparison of all 2400 mg cd patients versus placebo found an overall treatment effect (OR=2.9 (1.2 to 7.1), nominal p=0.02). Incidence of adverse events (AEs), serious AEs and infusion reactions was similar between epratuzumab and placebo groups, without decreases in immunoglobulin levels and only partial reduction in B-cell levels.
Conclusions: Treatment with epratuzumab 2400 mg cd was well tolerated in patients with moderately to severely active SLE, and associated with improvements in disease activity. Phase III studies are ongoing.
Keywords: B cells; Systemic Lupus Erythematosus; Treatment.
Figures
Similar articles
-
Efficacy and Safety of Epratuzumab in Moderately to Severely Active Systemic Lupus Erythematosus: Results From Two Phase III Randomized, Double-Blind, Placebo-Controlled Trials.Arthritis Rheumatol. 2017 Feb;69(2):362-375. doi: 10.1002/art.39856. Arthritis Rheumatol. 2017. PMID: 27598855 Free PMC article. Clinical Trial.
-
Interferon Inhibition for Lupus with Anifrolumab: Critical Appraisal of the Evidence Leading to FDA Approval.ACR Open Rheumatol. 2022 Jun;4(6):486-491. doi: 10.1002/acr2.11414. Epub 2022 Feb 14. ACR Open Rheumatol. 2022. PMID: 35157371 Free PMC article.
-
Efficacy of Epratuzumab, an Anti-CD22 Monoclonal IgG Antibody, in Systemic Lupus Erythematosus Patients With Associated Sjögren's Syndrome: Post Hoc Analyses From the EMBODY Trials.Arthritis Rheumatol. 2018 May;70(5):763-773. doi: 10.1002/art.40425. Epub 2018 Apr 12. Arthritis Rheumatol. 2018. PMID: 29381843 Free PMC article.
-
Evaluation of epratuzumab as a biologic therapy in systemic lupus erythematosus.Immunotherapy. 2014;6(11):1165-75. doi: 10.2217/imt.14.80. Immunotherapy. 2014. PMID: 25496332 Review.
-
Epratuzumab for the treatment of systemic lupus erythematosus.Expert Rev Clin Immunol. 2018 Apr;14(4):245-258. doi: 10.1080/1744666X.2018.1450141. Epub 2018 Mar 20. Expert Rev Clin Immunol. 2018. PMID: 29542345 Review.
Cited by
-
Current and emerging treatment options in the management of lupus.Immunotargets Ther. 2016 Mar 2;5:9-20. doi: 10.2147/ITT.S40675. eCollection 2016. Immunotargets Ther. 2016. PMID: 27529058 Free PMC article. Review.
-
B cells in systemic lupus erythematosus: Targets of new therapies and surveillance tools.Front Med (Lausanne). 2022 Aug 30;9:952304. doi: 10.3389/fmed.2022.952304. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36111105 Free PMC article. Review.
-
Meant to B: B cells as a therapeutic target in systemic lupus erythematosus.J Clin Invest. 2021 Jun 15;131(12):e149095. doi: 10.1172/JCI149095. J Clin Invest. 2021. PMID: 34128474 Free PMC article. Review.
-
Model-based meta-analysis using latent variable modeling to set benchmarks for new treatments of systemic lupus erythematosus.CPT Pharmacometrics Syst Pharmacol. 2024 Feb;13(2):281-295. doi: 10.1002/psp4.13083. Epub 2023 Dec 4. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 38050332 Free PMC article.
-
The need to define treatment goals for systemic lupus erythematosus.Nat Rev Rheumatol. 2014 Sep;10(9):567-71. doi: 10.1038/nrrheum.2014.118. Epub 2014 Jul 22. Nat Rev Rheumatol. 2014. PMID: 25048762 Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

