Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma
- PMID: 23313965
- PMCID: PMC3578068
- DOI: 10.1053/j.gastro.2013.01.002
Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma
Abstract
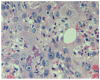
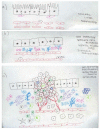
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and the third greatest cause of cancer-related death worldwide, and its incidence is increasing. Despite the significant improvement in management of HCC over the past 30 years, there are no effective chemoprevention strategies, and only one systemic therapy has been approved for patients with advanced tumors. This drug, sorafenib, acts on tumor cells and the stroma. HCC develops from chronically damaged tissue that contains large amounts of inflammation and fibrosis, which also promote tumor progression and resistance to therapy. Increasing our understanding of how stromal components interact with cancer cells and the signaling pathways involved could help identify new therapeutic and chemopreventive targets.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. - PubMed
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 61:69–90. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. - PubMed
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

