Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy
- PMID: 23314070
- PMCID: PMC3690323
- DOI: 10.1016/j.bone.2013.01.002
Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy
Abstract
Introduction: Heterotopic ossification (HO), or the abnormal formation of bone in soft tissue, occurs in over 60% of major burn injuries and blast traumas. A significant need exists to improve the current diagnostic modalities for HO which are inadequate to diagnose and intervene on HO at early time-points. Raman spectroscopy has been used in previous studies to report on changes in bone composition during bone development but has not yet been applied to burn induced HO. In this study, we validate transcutaneous, in-vivo Raman spectroscopy as a methodology for early diagnosis of HO in mice following a burn injury.
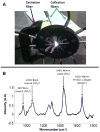
Methods: An Achilles tenotomy model was used to study HO formation. Following tenotomy, mice were divided into burn and sham groups with exposure of 30% surface area on the dorsum to 60° water or 30° water for 18s respectively. In-vivo, transcutaneous Raman spectroscopy was performed at early time points (5 days, 2 and 3 weeks) and a late time point (3 months) on both the tenotomized and non-injured leg. These same samples were then dissected down to the bone and ex-vivo Raman measurements were performed on the excised tissue. Bone formation was verified with Micro CT and histology at corresponding time-points.
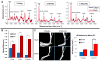
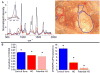
Results: Our Raman probe allowed non-invasive, transcutaneous evaluation of heterotopic bone formation. Raman data showed significantly increased bone mineral signaling in the tenotomy compared to control leg at 5 days post injury, with the difference increasing over time whereas Micro CT did not demonstrate heterotopic bone until three weeks. Ex-vivo Raman measurements showed significant differences in the amount of HO in the burn compared to sham groups and also showed differences in the spectra of new, ectopic bone compared to pre-existing cortical bone.
Conclusions: Burn injury increases the likelihood of developing HO when combined with traumatic injury. In our in-vivo mouse model, Raman spectroscopy allowed for detection of HO formation as early as 5 days post injury. Changes in bone mineral and matrix composition of the new bone were also evidenced in the Raman spectra which could facilitate early identification of HO and allow more timely therapy decisions for HO patients.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: None
Figures


References
-
- Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37:129–136. - PubMed
-
- Potter BK, et al. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92(Suppl 2):74–89. - PubMed
-
- Cassar-Pullicino VN, et al. Sonographic diagnosis of heterotopic bone formation in spinal injury patients. Paraplegia. 1993;31:40–50. - PubMed
-
- Freed JH, Hahn H, Menter R, Dillon T. The use of the three-phase bone scan in the early diagnosis of heterotopic ossification (HO) and in the evaluation of Didronel therapy. Paraplegia. 1982;20:208–216. - PubMed
-
- McCreadie BR, Goulet RW, Feldkamp LA, Goldstein SA. Hierarchical structure of bone and micro-computed tomography. Adv Exp Med Biol. 2001;496:67–83. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

