Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism
- PMID: 23314219
- PMCID: PMC3656360
- DOI: 10.1038/npp.2013.12
Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism
Abstract
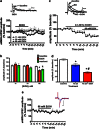
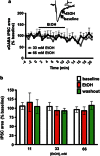
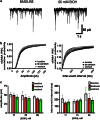
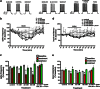
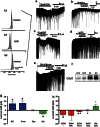
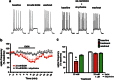
Trauma-induced damage to the orbitofrontal cortex (OFC) often results in behavioral inflexibility and impaired judgment. Human alcoholics exhibit similar cognitive deficits suggesting that OFC neurons are susceptible to alcohol-induced dysfunction. A previous study from this laboratory examined OFC mediated cognitive behaviors in mice and showed that behavioral flexibility during a reversal learning discrimination task was reduced in alcohol-dependent mice. Despite these intriguing findings, the actions of alcohol on OFC neuron function are unknown. To address this issue, slices containing the lateral OFC (lOFC) were prepared from adult C57BL/6J mice and whole-cell patch clamp electrophysiology was used to characterize the effects of ethanol (EtOH) on neuronal function. EtOH (66 mM) had no effect on AMPA-mediated EPSCs but decreased those mediated by NMDA receptors. EtOH (11-66 mM) also decreased current-evoked spike firing and this was accompanied by a decrease in input resistance and a modest hyperpolarization. EtOH inhibition of spike firing was prevented by the GABAA antagonist picrotoxin, but EtOH had no effect on evoked or spontaneous GABA IPSCs. EtOH increased the holding current of voltage-clamped neurons and this action was blocked by picrotoxin but not the more selective GABAA antagonist biccuculine. The glycine receptor antagonist strychnine also prevented EtOH's effect on holding current and spike firing, and western blotting revealed the presence of glycine receptors in lOFC. Overall, these results suggest that acutely, EtOH may reduce lOFC function via a glycine receptor dependent process and this may trigger neuroadaptive mechanisms that contribute to the impairment of OFC-dependent behaviors in alcohol-dependent subjects.
Figures

References
-
- Adermark L, Clarke RB, Olsson T, Hansson E, Soderpalm B, Ericson M. Implications for glycine receptors and astrocytes in ethanol-induced elevation of dopamine levels in the nucleus accumbens. Addict Biol. 2011;16:43–54. - PubMed
-
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-Methyl-D-aspartate receptor function. J Biol Chem. 2003;278:11020–11025. - PubMed
-
- Anders DL, Blevins T, Sutton G, Chandler LJ, Woodward JJ. Effects of c-Src tyrosine kinase on ethanol sensitivity of recombinant NMDA receptors expressed in HEK 293 cells. Alcohol Clin Exp Res. 1999;23:357–362. - PubMed
-
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

